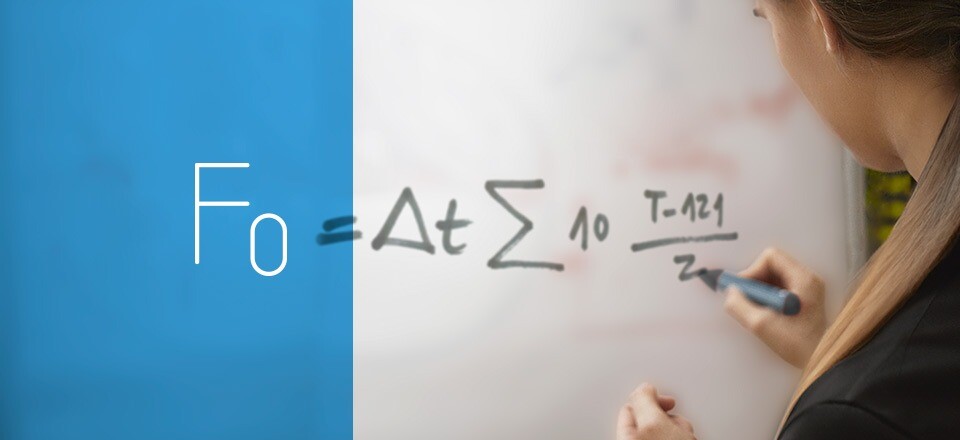Which are the sterilization cycles of an autoclave?
Discover the main sterilization cycles of an autoclave and how to choose the right one according to the type of instruments to be treated.

The sterilization process utilizing an autoclave employs the principle of steam application under elevated pressure and temperature to achieve microbial inactivation. However, it is crucial to recognize that sterilization cycles are not universally applicable; each cycle is tailored for specific applications. The selection of the appropriate cycle is contingent upon the nature of the instruments or materials subjected to sterilization.
This article delves into the most prevalent sterilization cycles employed in autoclaves, providing a simplified exposition to facilitate informed decision-making in selecting the optimal cycle for your specific sterilization requirements.
Autoclave sterilization cycles
1. Gravity cycle
The gravity cycle, also known as the gravity displacement cycle, represents the most fundamental and traditional method of autoclave sterilization. In this cycle, steam displaces the air in the chamber by gravity, with the denser cold air being pushed out as the lighter steam rises to the top of the sterilization chamber. As steam fills the chamber, the air is expelled through a drain vent. This displacement allows the steam to make direct contact with the load, initiating the sterilization process. The gravity cycle is ideal for sterilizing simple solid objects and is a standard function in all autoclaves. Its straightforwardness, efficiency, and dependability make it a popular choice for sterilization. Learn more about the gravity cycle.
2. Vacuum cycle
The vacuum cycle, commonly referred to as the prevacuum cycle, marks a pivotal advancement in sterilization technology. This method utilizes a vacuum pump to remove all cold air from the chamber prior to the introduction of steam, thereby achieving enhanced sterilization efficacy. There are two principal variants of this method: the single prevacuum cycle and the fractionated prevacuum cycle. Both variants fundamentally rely on the mechanical extraction of cold air using a vacuum pump, ensuring a more thorough and consistent sterilization process. This approach is particularly effective and essential for sterilizing textiles, porous materials, bagged items, and hollow objects with complex geometries. Learn all the details about the vacuum cycle.

3. Liquids cycle
If you need to sterilize liquids, this is your optimal choice. Derived from the gravity cycle, this process is distinguished by its careful regulation of pressure and temperature during the cooling phase to prevent splashes, container breakage, or evaporation losses of the load. It is the recommended cycle for sterilizing bottles with culture media or aqueous solutions. Learn more about the liquids cycle.
4. Cycle with drying
Are you seeking to ensure that your instruments are completely dry at the conclusion of the sterilization cycle? Are you weary of the inefficiency and inconvenience of having to dry wet loads in an external oven post-sterilization? The cycle with drying provides an optimal solution by integrating an additional drying phase following the cooling phase. This enhancement effectively eliminates all residual moisture, rendering your glassware and instruments perfectly dry. This cycle is particularly advantageous for sterilizing items that necessitate complete dryness. Additional information about the cycle with drying.
5. Cycle with fast cooling
When time is of the essence, the cycle with fast cooling emerges as the optimal solution to enhance productivity and efficiency. This cycle significantly accelerates the cooling of sterilized items, substantially shortening the duration of the cooling phase and thereby improving both process efficiency and safety. It is particularly well-suited for laboratories with high instrument turnover or for those requiring frequent sterilization of liquid loads. Learn more about the cycle with fast cooling.
6. Isothermal or low-temperature cycle.
The isothermal or low-temperature cycle is specifically designed for processing thermolabile materials. This cycle employs lower temperatures combined with extended exposure times to effectively pasteurize or sterilize loads without compromising their chemical composition, altering the structural integrity of electronic components, or damaging delicate materials. Discover here all the details about the isothermal cycle and how it can meet your sterilization needs while preserving the integrity of sensitive items.
7. Flash cycle
The flash cycle serves as a critical tool in emergency situations, offering rapid sterilization for instruments that require immediate reuse. Despite its high efficiency, the use of this cycle demands caution due to its dependence on shorter exposure times and less rigorous pre-vacuum processes. Learn more about the flash cycle.
8. F₀ cycle
The F₀ cycle is an extensively utilized technique for quantifying and optimizing microbial inactivation in sterilization processes, while ensuring that the processed load is not exposed to excessive thermal stress. This method is based on the principle of thermal lethality, employing precise calculations to achieve effective microbial eradication. Unlike other sterilization cycles that regulate exposure time solely through chamber temperature, the F₀ cycle uses F values, a parameter that accounts for the accumulated heat transfer to the load. This method is primarily employed by professionals in highly regulated sectors and is considered the gold standard in the food and pharmaceutical industries due to its superior accuracy in controlling and quantifying sterilization. Learn all the details about the F₀ cycle.

9. Cycles with pressure support
Cycles with pressure support are indispensable for the processing of hermetically sealed containers and are highly recommended for the sterilization of semi-open liquid-filled containers, particularly where liquid retention is paramount, such as in prefilled syringes. There are two principal variants of cycles with pressure support: the air over-pressure cycle and the steam-air-mix cycle. Each is meticulously engineered to address distinct requirements and accommodate various load types. Both cycles employ an air compressor to apply additional pressure during the sterilization and/or cooling phases, thereby reducing the risk of liquid loss due to evaporation or container rupture. This approach is especially beneficial for the sterilization of food products and pharmaceuticals. Learn more about the specific functions and benefits of cycles with pressure support.
10. Cycle with temperature segments
The cycle with temperature segments is a specialized and infrequently employed sterilization protocol, predominantly utilized in research and industrial settings for processing products necessitating gradual and meticulously regulated thermal treatment. This advanced cycle facilitates the programming of discrete temperature segments and the precise control of the rate of temperature and pressure transition between these segments. The protocol encompasses multiple stages within both the heating and cooling phases, enabling a high degree of customization. Its diverse applications span from the production of specific culture media formulations in microbiological laboratories to the functional assessment of electronic components that require delicate thermal conditioning prior to sterilization. Additionally, it finds use in food processing scenarios where raw food items undergo cooking followed by sterilization. Learn more about the cycle with temperature segments.
11. Accelerated aging cycle
For those who aim to study and validate the durability and resilience of their products, the accelerated aging cycle is an invaluable tool. This cycle replicates extreme environmental conditions to predict long-term performance within a shortened timeframe. Autoclaves with this capability allow for the programming of extended exposure durations and multiple consecutive sterilization cycles. Utilized extensively in research and quality control laboratories within the industrial and pharmaceutical sectors, this cycle ensures that products meet stringent durability standards. Learn more about the accelerated aging cycle.
Evaluation tests for autoclaves
Vacuum test
The vacuum test is an essential functional evaluation specifically designed for autoclaves equipped with vacuum cycle capabilities. This test rigorously assesses both the performance of the autoclave’s vacuum system and the integrity of its hermetic sealing. Ensuring the autoclave can achieve and maintain a sufficient vacuum is critical. This test cycle is particularly indispensable for laboratories that routinely employ vacuum cycles to sterilize bagged items, instruments with internal cavities, and porous materials. Learn more about the vacuum test.
Bowie & Dick Test
The Bowie & Dick test is a critical diagnostic tool used to verify the effective penetration of steam into porous materials during sterilization processes. This control test is extensively employed in healthcare environments to assess the performance of an autoclave’s vacuum system and the quality of the employed steam. It is crucial for preventing any inadvertent compromises in sterilization efficacy. Learn all the details of the Bowie & Dick test.

Helix test
The Helix test evaluates the autoclave’s ability to sterilize hollow instruments with complex cavities and geometries, ensuring that steam efficiently penetrates into all corners of the instruments. It is a control test associated with a specific sterilization cycle commonly used in operating rooms and dental clinics to validate the effectiveness of autoclaves in the sterilization of laparoscopic instruments or dental cannulas. More information about the Helix test.
In a nutshell
The selection of an appropriate sterilization cycle is crucial to ensuring the thorough sterilization of laboratory instruments and materials without inducing undesirable structural damage. As previously discussed, each type of cycle offers specific advantages based on the material and its intended use. To gain a comprehensive understanding of these sterilization cycles and determine the most suitable one for your needs, we encourage you to explore the links provided throughout this post.
At RAYPA, we are committed to excellence in sterilization and we offer you the best technology to ensure maximum safety and efficiency in your processes. If you have any questions, do not hesitate to reach out to our experts. Working together, we can identify and provide a solution that ensures both efficiency and safety in all your sterilization operations.