The autoclave sterilization process
Familiarize yourself with the autoclave sterilization process, the most widely used and effective method for inactivating microorganisms in scientific and medical settings.

In the fields of science and medicine, sterilization is a fundamental process to ensure that instruments and components used in medical procedures, tests, and experiments are entirely free of living microorganisms.
Historically, sterilization has been achieved through the use of dry heat, which is effective in denaturing proteins and eradicating microorganisms. However, the direct application of fire for sterilization is impractical for most materials, as it alters their physicochemical properties. However, the direct application of fire for sterilization is impractical for most materials, as it alters their physicochemical properties. Additionally, its hazardous nature poses significant risks in any laboratory setting. These limitations have driven the development and adoption of more sophisticated and safer sterilization methods.
The autoclave: The most popular sterilization method
Among the various sterilization techniques available, steam autoclaving has become the preferred method in laboratories for a wide range of applications due to its effectiveness, convenience, low cost, safety, and absence of toxicity risks. This process, known as autoclaving, is capable of inactivating bacteria, viruses, fungi, and even prions. Despite the existence of alternative methods such as hot air ovens, ethylene oxide, or irradiation, steam autoclaving is widely recognized for its efficacy and versatility. However, it is not suitable for processing heat-sensitive materials, as they may undergo irreversible physicochemical changes under these conditions.
Fundamentals of autoclave sterilization
The process of sterilization using an autoclave is a scientifically validated method that employs saturated steam under high pressure to achieve and maintain elevated temperatures, capable of eradicating both microorganisms and their spores.
The operation begins with the placement of items inside the autoclave chamber, which is then hermetically sealed. Once the cycle is initiated, the machine evacuates the air from the chamber to allow the subsequent introduction of saturated steam, ensuring complete and uniform heat transfer to all surfaces of the load.

The standard sterilization temperature of 121 degrees Celsius is effective against most microorganisms and is the default setting for many sterilization cycles. However, for processing food or heat-sensitive materials that could be damaged at this temperature, such as certain plastics or solutions that might denature, an isothermal cycle with a lower temperature of 105 degrees Celsius is used.
In contrast, surgical instruments, which require an extremely high degree of sterility due to their use in invasive procedures, are typically subjected to higher temperatures, up to 134 degrees Celsius, to ensure the destruction of particularly resistant pathogens, such as the most robust bacterial spores or prions.
Safety in the cooling phase
After the sterilization phase, the autoclave transitions to a cooling stage, where the temperature and internal pressure are gradually reduced to match ambient conditions. This step is crucial for the safety of the process, as premature opening of the chamber could result in the rapid release of hot, pressurized steam, posing a risk of burns and other workplace accidents. Additionally, gradual cooling helps prevent thermal shock to the sterilized materials, which could cause structural damage to the load or excessive condensation of steam on the load.
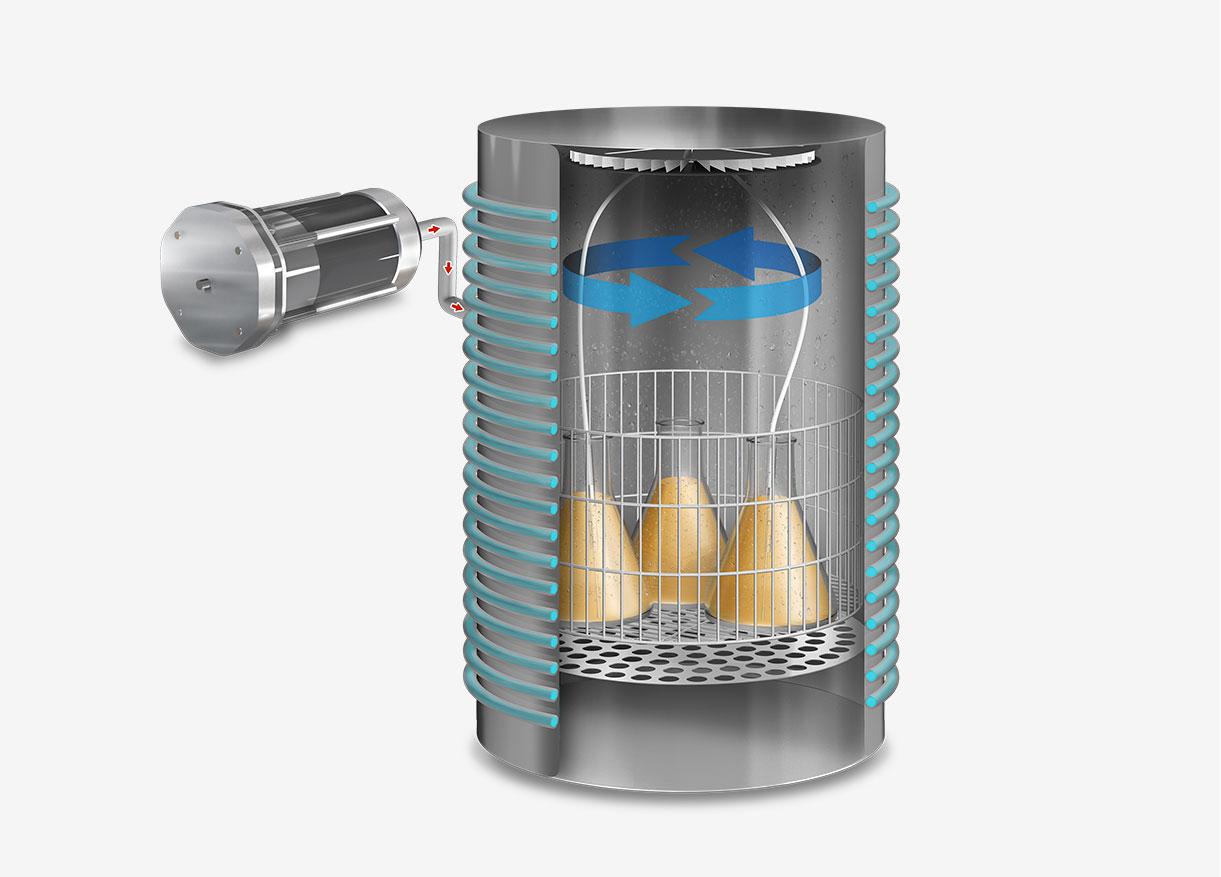
Once the pressure and temperature reach safe levels, the more advanced autoclave models initiate a vacuum drying cycle. This part of the cycle is essential for porous loads because any residual moisture in the sterilized items could not only serve as a breeding ground for microorganisms but also compromise the integrity of subsequent procedures in which the materials are used, such as cell cultures or pharmaceutical production.
Time and temperature: Key factors in sterilization
The duration of the sterilization cycle is a fundamental component for the effectiveness of the process. Reaching the target temperature alone is insufficient; it is imperative to maintain this temperature and pressure for an adequate period to ensure complete heat penetration and subsequent sterilization of the items.
The time required for effective sterilization varies based on the size and density of the load, as well as the nature of the materials to be sterilized. Generally, a period of at least 20 minutes at 121 degrees Celsius is sufficient to sterilize most objects. However, for larger or denser loads, or when sterilizing objects with more complex geometries, this time may need to be extended.
During this period, any water and moisture present in the chamber is completely evaporated, ensuring that at the end of the cycle, the items are thoroughly dry and free of any form of microbial contamination, thus effectively completing the sterilization process.
Additionally, the efficiency of the autoclave sterilization process also depends on the correct preparation and packaging of the materials before sterilization. Materials must be cleaned and, if necessary, disinfected before being placed in the autoclave to ensure that the steam can contact all surfaces effectively. The packaging must allow steam penetration to all surfaces of the load while also protecting the items from environmental contamination after sterilization.

Another essential factor is the continuous training of personnel in good sterilization practices. Regular training helps to foster and maintain competence in these critical procedures and to adapt to technological advances that may influence sterilization techniques and infection control protocols.
The meticulous nature of the autoclave sterilization process ensures its reliability and effectiveness, making it a fundamental pillar in infection prevention in clinical settings and the preservation of the integrity of scientific experiments.
Sterilization not only involves the elimination of microorganisms but also the maintenance of sterility over time. Once the items have been sterilized and dried in the autoclave, they must be handled and stored in a manner that preserves their sterile condition. This generally requires the use of sterile wrappings and aseptic handling techniques, as well as the implementation of storage protocols that protect against environmental contamination and microbial proliferation.
Preparation and packaging of items before autoclave sterilization
Proper preparation of materials before introducing them into the autoclave is essential to ensure effective sterilization. Items must be meticulously cleaned to remove any biological or chemical residues that could shield microorganisms from the heat of the steam. Additionally, the packaging and arrangement of materials inside the autoclave must allow for free circulation of the steam.
The use of special porous packaging materials and proper sealing are standard practices that facilitate this process, ensuring that the items remain sterile until they are used.
Maintenance and verification of autoclave operation
Regular maintenance of autoclaves is crucial to ensure their optimal functioning, safety and efficacy of the sterilization process. This maintenance includes periodic calibration of temperature and pressure sensors, as well as thorough inspection of seals and safety valves to prevent steam leaks and loss of efficacy. Additionally, it is important to regularly accompany each cycle with biological and chemical control tests to verify the effectiveness of the sterilization process. These controls are essential for identifying any potential failures in the process that could compromise the sterility of the processed materials.
Continuous training and updating sterilization protocols
Ongoing training of personnel responsible for operating autoclaves is an essential component for ensuring the success of the sterilization process. Operators must stay current with the latest advancements in sterilization techniques as well as technological innovations that may impact these procedures. It is crucial that they thoroughly understand the specific protocols for sterilizing various types of materials and loads, ranging from surgical instruments to culture media.
Concurrently, sterilization protocols must be continuously reviewed and improved to reflect best practices and the latest international standards. This updating process should include a critical evaluation of existing procedures, integration of new scientific evidence, and incorporation of emerging technologies. Additionally, effective communication of these changes to personnel is vital to ensure a smooth transition and effective implementation of the new protocols.
Beyond technical training, it is imperative that operators are trained in the identification and management of equipment failures. This includes the ability to detect signs of malfunction, such as fluctuations in temperature or pressure, and to implement immediate corrective measures to prevent contamination of materials. Training should also cover aspects of quality management and meticulous documentation of each sterilization cycle, thereby ensuring traceability and compliance with established regulations.