The Autoclave Spore Test: What is it and why is it important?
Ensure effective sterilization with the autoclave spore test. Learn how to perform it and download your free log sheet for complete monitoring.
The autoclave spore test is a crucial procedure for ensuring rigorous sterilization control in both healthcare and research settings, including hospitals, tattoo studios, clinical analysis centers, and the microbiology industry, among others.
This method is considered the gold standard for assessing whether a sterilization process successfully eliminates all microorganisms, including the most resistant ones, such as bacterial spores.
In this article, you will learn how to perform the autoclave spore test, understand the importance of spore control, and explore key regulations to maintain high levels of safety and quality.
What is the Spore Test?
The spore test is a biological indicator used to evaluate the effectiveness of a sterilization process.
Unlike chemical or physical indicators, the spore test uses highly resistant bacterial spores, such as those of Geobacillus stearothermophilus, which are subjected to high-pressure steam sterilization cycles.
If the cycle is effective, the spores are destroyed, indicating a successful sterilization process. Conversely, if the spores survive, it reveals a sterilization failure, posing a significant risk to patient and user safety.
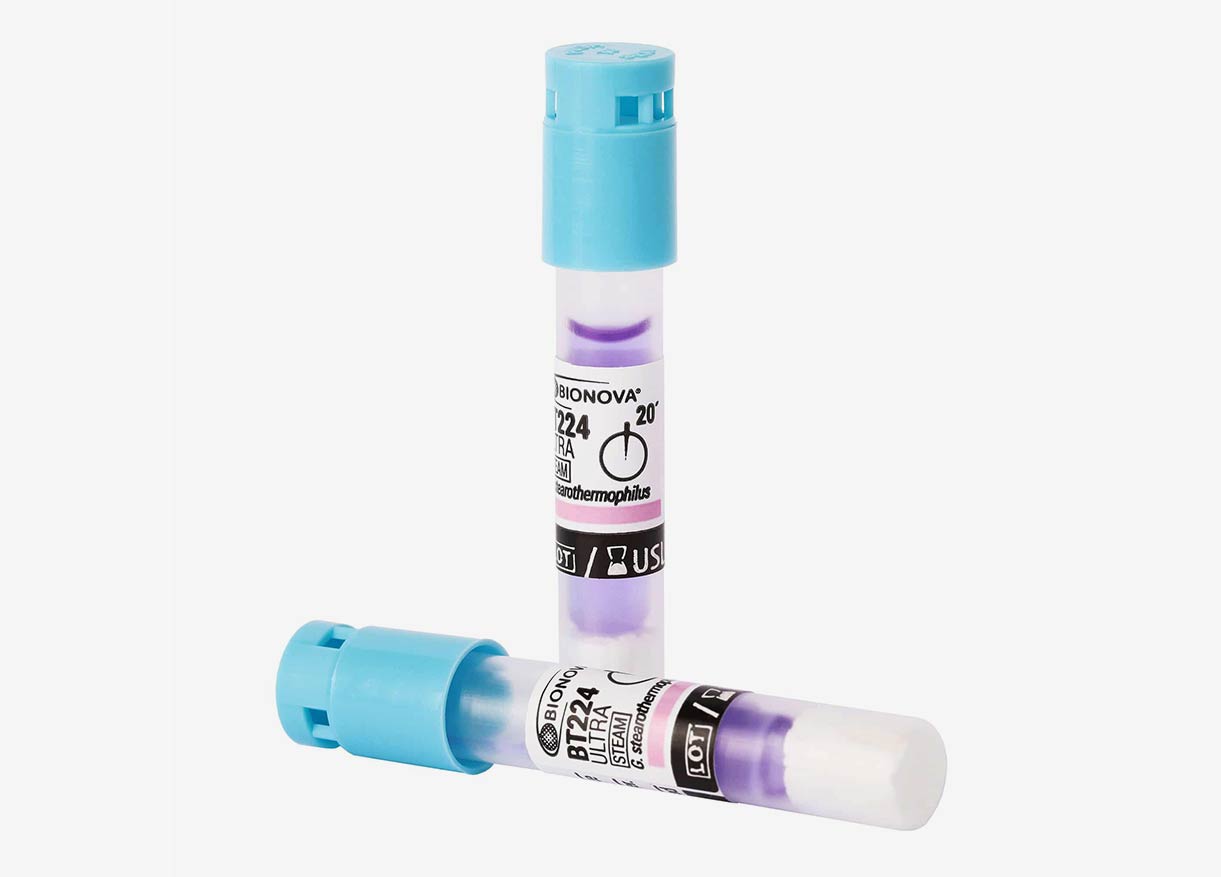
Why is the Autoclave Spore Test Important?
Spore testing in autoclaves is crucial because it provides the most accurate verification of whether equipment has achieved complete sterilization.
Other methods, such as chemical indicators, only confirm if the appropriate parameters for time, temperature, and pressure have been met but do not verify whether the most resistant bacteria have been eliminated.
Routine use of this test is mandatory in many clinical and aesthetic environments. International regulations, such as those from the Centers for Disease Control and Prevention (CDC) and European guidelines, recommend regular spore testing, particularly for critical cycles involving surgical instruments or implants.
How to Perform the Autoclave Spore Test
Performing the autoclave spore test is relatively straightforward but requires strict adherence to a protocol to ensure its effectiveness. Below are the key steps:
1. Preparation of the Biological Indicator
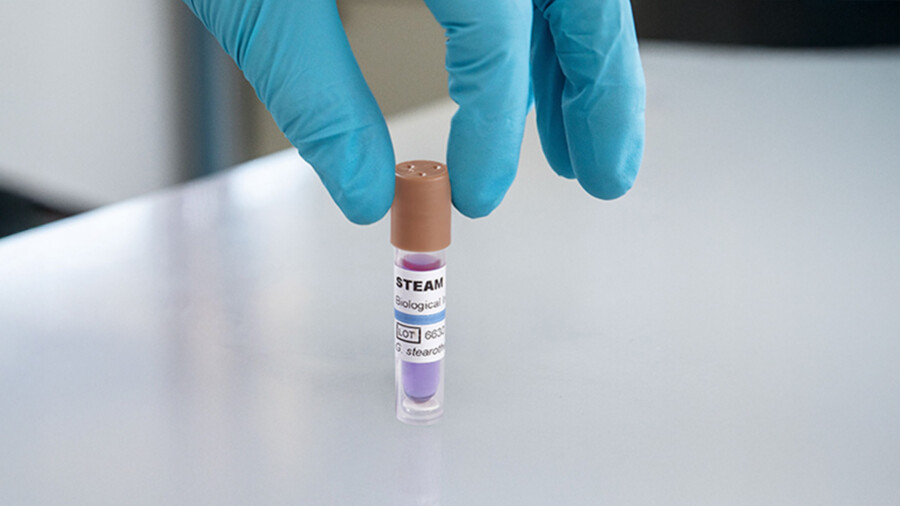
Biological indicators are small vials, strips, or ampoules containing a controlled amount of resistant bacterial spores. These spores are suspended in a medium that promotes their growth if they survive the sterilization process.
The first step is to place the vial, strip, or ampoule inside a bag representative of the materials to be sterilized.
2. Placement in the Autoclave
The vial or strip containing spores should be placed in the least accessible areas of the autoclave, such as the corners of the sterilization chamber or near the drain, especially in gravity displacement autoclaves.
In fractionated vacuum autoclaves, steam distribution tends to be more uniform, but it is still essential to position the biological indicators in these critical areas. These locations are more likely to be cold spots or have reduced steam penetration, making them crucial for verifying the effectiveness of the cycle.
3. Execution of the Sterilization Cycle
Once the biological indicators are positioned, a standard sterilization cycle is run.
It is important to follow the autoclave manufacturer’s instructions, ensuring the correct temperature, time, and pressure parameters for each cycle type.
4. Incubation of the Biological Indicator
After the cycle is complete, the vial is removed and placed in an incubator at a temperature between 55 and 60°C for 24 to 48 hours, depending on the type of incubator (some rapid incubators can provide results in just a few hours).
During this process, the survival and growth of the spores are monitored, typically indicated by a color change in the growth medium.
5. Interpretation of Results
Results are assessed visually or with a rapid incubator that analyzes the vial’s colorimetry.
A yellow color indicates that the spores survived, meaning the sterilization cycle failed.
If the color remains purple or there is no growth, it confirms that sterilization was successful.
How Often Should the Autoclave Spore Test Be Performed?
International regulations recommend conducting autoclave spore testing weekly, particularly for loads containing critical materials such as surgical implants.
It is also advised to perform the test during the first sterilization cycle of each day or whenever there is suspicion that the autoclave may not be functioning correctly.
In some healthcare sectors, regulations mandate more frequent testing, such as daily cycles or immediately after autoclave repairs.
Non-medical facilities, such as tattoo studios or beauty salons, may be subject to different regulations. However, following similar practices is highly recommended to ensure safety and compliance.
Differences Between the Spore Test and Other Sterilization Controls
It is essential to understand the differences between biological indicators and other types of controls, such as chemical indicators.
While chemical indicators are faster and more economical, they do not guarantee that sterilization was effective. They only confirm that certain parameters, like temperature and time, were achieved during the cycle.
The autoclave spore test, on the other hand, provides a definitive verification that all microorganisms, including resistant spores, have been eliminated.
Chemical Indicators
There are various classes of chemical indicators, ranging from the simplest (Class 1), which only indicate if a package has been through the autoclave, to more advanced ones (Class 5 or 6), which measure multiple parameters like temperature and time.
However, while these indicators can detect cycle failures, they do not offer the same level of assurance as a biological indicator and cannot serve as substitutes.
Biological Indicators
The spore test is the only indicator capable of confirming the complete elimination of microorganisms.
This type of control is especially critical in environments where material sterilization must be absolutely reliable, such as in the sterilization of surgical instruments or the preparation of dental implants.
International Standards and Recommendations
Various health organizations and international standards, such as ISO guidelines and CDC recommendations, emphasize the importance of performing periodic biological testing on autoclaves.
ISO 11140 and ISO 11138 regulate the requirements for chemical and biological indicators, respectively, while the CDC recommends using bioindicators as part of an infection control protocol.
Additionally, some European countries and U.S. states mandate that spore testing be performed at least weekly, and in some cases, even more frequently to ensure patient safety.
Protocol for Handling a Spore Test Failure
If the autoclave spore test yields a positive result (spore growth), immediate action must be taken.
The first step is to remove the instruments sterilized during that cycle from use and repeat the sterilization process.

Additionally, it is essential to inspect the autoclave, recalibrate the equipment if necessary, and diagnose potential mechanical or programming issues.
Once the problem has been resolved, it is recommended to perform a second spore test to confirm that the autoclave is functioning correctly before putting it back into operation.
In a nutshell
The spore test is an essential tool for ensuring that autoclave sterilization processes are effective and safe.
While it may seem like an additional procedure, its regular implementation is critical to preventing infection risks and ensuring safety in clinics, hospitals, and other settings.
Following recommendations on how to perform the autoclave spore test and maintaining proper spore control is key to creating a safe, contamination-free working environment.
This article has covered the most important aspects of the autoclave spore test, from its significance to its proper implementation.
By following these steps, any facility using autoclaves can ensure effective sterilization processes that comply with the strictest infection control regulations.
Download Your Spore Test Log Sheet
Keeping accurate records is essential to ensure sterilization process traceability and comply with infection control regulations in clinical settings. To help you track autoclave spore test results, we’ve created a log sheet you can download for free.