Sterilization cycle with temperature segments: everything you need to know
Explore how the sterilization cycle with temperature segments provides increased flexibility, boosting research capabilities across various sectors. Discover all the details about its application in this article.

The sterilization cycle with temperature segments or ramping represents an advanced technique employed in specialized research fields such as microbiology, food technology, and packaging development. This strategy is employed for the safe production of food, the validation of the structural integrity of packaging, and the assessment of the robustness of innovative materials, while ensuring the efficient eradication of microbial contaminants.
Unlike the flash sterilization cycle, which is characterized by its rapidity and high temperatures, this process involves a sequence of stages at varying temperatures and pressures, carefully adjusted in both the pre-sterilization and post-sterilization stages.
What does sterilization with temperature segments involve?
This sterilization method is ideal for conducting tests that evaluate how temperature and pressure impact the physicochemical properties of a product. Such tests are particularly significant in research environments aiming to design new products, in quality control stress tests, and in the creation of pilot batches in the food industry.
To perform these tests, it is essential to have an autoclave integrated with vacuum and air compression systems, which facilitate the precise modulation of temperature and pressure conditions throughout the cycle. This capability is especially relevant in research environments, quality control, and stress testing.
Stages of the sterilization cycle with temperature segments
Typically, the cycle consists of several stages, each adjusted to specific parameters of duration, pressure, and temperature. In more specialized autoclaves, the rate at which temperature and pressure increase between segments can also be adjusted. The main phases are as follows:
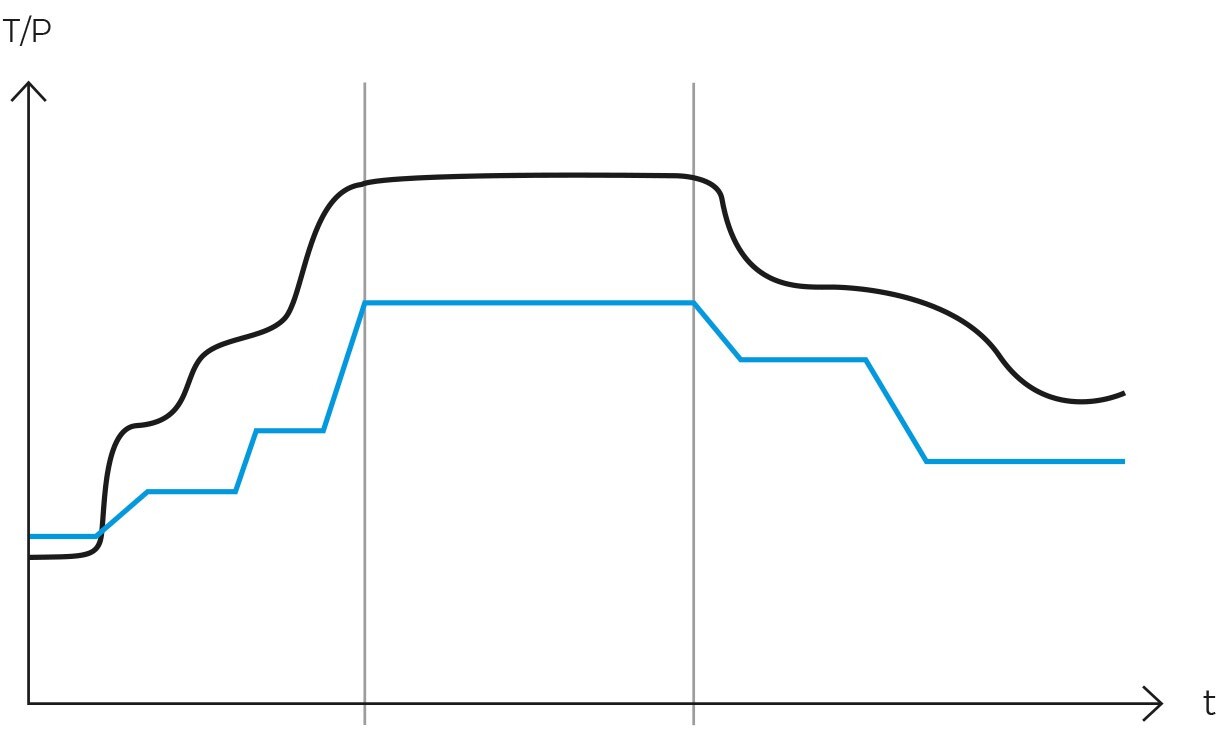
-
Prevacuum phase
Initially, air is removed from the chamber to ensure complete steam penetration. This stage is crucial for sterilizing porous loads, objects with cavities, and instruments with complex geometries.
-
Heating phase with temperature segments
Next, the heating phase begins, where the cycle is divided into several segments or ramps, each with a specific set of conditions for duration, pressure, and temperature. These segments are designed to gradually heat the load and prepare it for the sterilization phase. The transition between each segment may include stabilization periods to ensure that each change in conditions occurs in a controlled manner.
-
Sterilization phase
At this point, temperature and pressure are stabilized for a predetermined time to ensure the complete sterilization of all objects within the chamber.
-
Cooling phase with temperature segments
In the final stage, temperature and pressure are gradually reduced to return to normal conditions, preparing the objects for safe removal from the chamber. Optionally, this stage can also be structured into several segments, each with specific conditions of pressure and temperature.
These segments are designed to control the gradual reduction of the chamber’s temperature. Similar to the heating phase, the transition between these segments can include stabilization stages to ensure that the cooling of the load is controlled and uniform.
Importance of sterilization cycles with multiple segments

As previously mentioned, these cycles are ideal for researchers and experienced users, as they allow for detailed programming by time, pressure, and temperature. This ensures precise adaptation to the specific needs of each application. Additionally, these segments can be incorporated both before and after the sterilization phase, offering exceptional flexibility in process management. Below, we describe some examples of industries that utilize these types of cycles.
In the food packaging industry, cycles with segments are useful for several reasons, particularly for researchers and production operators handling plastic trays and other sensitive materials.
They are also widely used in the food industry and catering when cooking segments are preferred before reaching the sterilization temperature. This method allows raw foods to be cooked and sterilized within the same process. Popular applications include the preparation of sautéed foods where the semi-cooked preparation is packaged, or the cooking of meat and vegetables.
In microbiology, sterilization cycles with temperature segments are used for the preparation of special culture media containing thermolabile substances. In this process, the culture medium is first sterilized, then cooled, followed by the injection of antibiotics or thermolabile nutrients, and finally, the temperature is increased again to pasteurize the preparation.
A classic example is the preparation of blood agar. This process requires initial sterilization at 121°C for 15 minutes, cooling to 50°C, injecting the blood, pasteurizing for 15 minutes at 72°C, and then cooling again to 45°C.
Key considerations of sterilization with temperature segments
To ensure the effectiveness of sterilization with multiple temperature segments, it is essential to consider:
- Autoclave technology and components: ensure that the autoclave being used possesses the appropriate technology and components to perform these cycles.
- Material quality and preparation: monitor the quality and adequate preparation of the materials to be sterilized.
- Qualification after installation and annual calibration: calibrate the equipment annually and strictly follow the preventive maintenance plan established by the manufacturer.
- Operational and safety procedures: maintain a strict adherence to operational and safety procedures.
- User training: train all users who will handle the autoclave.
- Sterilization process validation: validate the sterilization process through the use of biological and chemical indicators.
Conclusions
The sterilization cycle with multiple segments or ramps is established as an advanced methodology, crucial for specialized applications in fields such as microbiology, the food industry, and packaging. This process, characterized by its sequence of controlled temperature and pressure stages, significantly differs from traditional sterilization methods due to its flexibility to meticulously adapt to the specific needs of different products and materials.
Although its implementation may be more complex than traditional methods, the ability to customize sterilization conditions makes it an invaluable tool for a wide range of applications in research and quality control.