FDA 21 CFR Part 11 compliance
Learn how to comply with FDA 21 CFR Part 11 regulations and ensure that your electronic records are secure and reliable. Read the article for more detailed information.

Since 1974, RAYPA has been synonymous with innovation and quality, offering products designed to meet the demands of critical sectors such as the pharmaceutical and biotechnology industries. To address the specific needs of this market segment, we introduced our Top Line autoclaves, which not only comply with FDA 21 CFR Part 11 regulations but also incorporate process optimization technology to deliver superior performance and efficiency.
In this article, we will delve into the significance of FDA 21 CFR Part 11 regulations in the context of autoclaves and highlight how our digital quality management systems ensure compliance with these regulations, establishing us as a benchmark of excellence in the laboratory autoclave sector.
What is the FDA?
The United States Food and Drug Administration (FDA) is a federal agency under the Department of Health and Human Services. Its primary role is to protect public health by regulating and supervising the safety, efficacy, and quality of human and animal drugs, biological products, medical devices, food, cosmetics, and products that emit radiation.
Additionally, the FDA is responsible for implementing and overseeing compliance with regulations governing the manufacturing, marketing, and distribution of these products, ensuring that the information provided to consumers is accurate and clear. With a broad range of responsibilities, the FDA plays a crucial role in monitoring advancements in medical and technological sciences, as well as in innovating food safety and public health protection.

The importance of traceability in FDA-regulated environments
Traceability, defined as the ability to track any product through all stages of its research, development, production, distribution, and use, is a critical aspect in the pharmaceutical and biotechnology industries. In a sector where transparency, reproducibility, and safety are paramount, ensuring that products meet the highest quality standards is essential.
In this context, the FDA has developed a specific regulatory framework, known as FDA 21 CFR Part 11, to regulate the minimum mandatory digital traceability requirements in all processes related to food, drugs, and medical devices. These regulations mandate that pharmaceutical and biotechnology companies maintain exhaustive and indelible records of every process to monitor and evaluate quality and to trace and address any potential issues that may arise.
Within this framework, FDA 21 CFR Part 11 stands out by setting specific requirements for electronic records, electronic signatures, and audit trails. These elements are essential to ensuring the integrity of traceability, facilitating accurate, secure, and reliable tracking throughout the entire product lifecycle.
What is FDA 21 CFR Part 11?
The FDA 21 CFR Part 11 regulation, established by the FDA in 1997 as part of its Code of Federal Regulations, aims to promote the use of electronic technology in place of paper for all records, ensuring data integrity and guaranteeing that information contained in electronic records is as reliable and valid as paper records and signatures. This regulation is particularly relevant and mandatory in the pharmaceutical, life sciences, and food sectors.
This regulation enhances data management by ensuring reliable storage, processing, and retrieval, encouraging the adoption of electronic records, electronic signatures, and audit trails.
- Electronic Records: A combination of text, graphics, data, images, or audio represented in digital format—equivalent to a paper record—created, modified, and archived by a computer system.
- Electronic Signatures: A combination of letters and symbols representing the user’s name and password. These not only store the name and date but also the meaning of the signature, whether for review, approval, or rejection. Electronic signatures help maintain uniqueness, facilitate periodic reviews, and prevent unauthorized actions.
- Audit Trails: Electronic records that allow the complete reconstruction of a sequence of events related to the creation, modification, or deletion of electronic records. These must be attributable, legible, contemporaneous, original, and accurate, collecting detailed information about the user, the action performed, and the date and time it was performed.
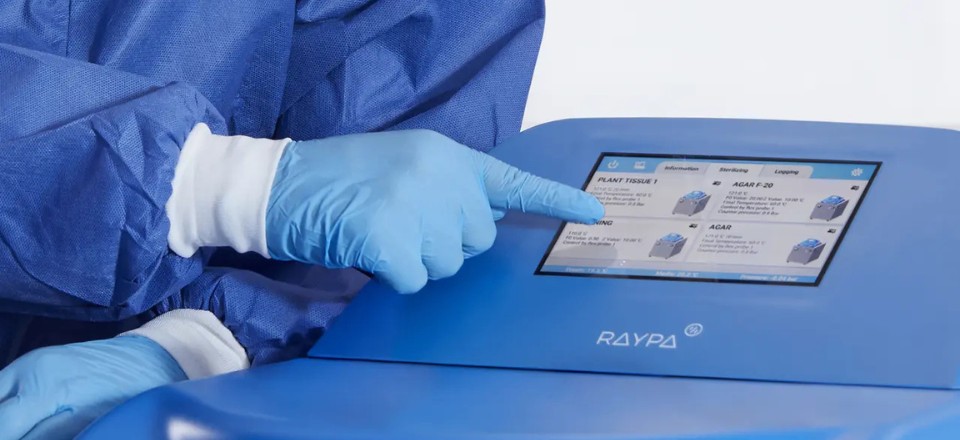
RAYPA Top line autoclaves: guaranteed compliance with FDA 21 CFR Part 11
For companies and entities seeking an autoclaves that adheres to this standard, our Top line autoclaves are ideal. They not only incorporate top-tier sterilization technology but also offer advanced technical support and professional traceability. Our Top line autoclaves are equipped with the most advanced connectivity on the market and are designed to operate under FDA, GMP, and GLP environments.

Top line autoclaves are an all-encompassing solution that redefine performance, efficiency and traceability through:
- RAYPACONTROL: Advanced cycle programming, real-time program visualization, admin user control, and complete access to protocol history.
- RAYPACLOUD: An integrated web-based management platform that includes personalized reports, integrated device management, alerts, audit traceability, and SSL encryption. It also offers integration possibilities within private servers, LIMS, or Active directory.
- RAYPASUPPORT: A set of functions that streamline maintenance management, offering remote equipment status monitoring, and remote diagnostics by the manufacturer and authorized technicians.
Alongside these functionalities, we offer IQ/OQ/PQ qualification services for the autoclave, IQ/OQ qualification for FDA compliance of the controller, and IQ/OQ qualification for FDA compliance of the management software in private installation modes. Additionally, these autoclaves can be complemented with a set of accessories that elevate your quality standards in accordance with Good Laboratory Practices (GLP), such as a label printer and barcode scanner.
Ensure compliance with FDA 21 CFR Part 11 on your sterilization processes with our autoclaves.