F0 sterilization cycle
Discover how sterilization cycles governed by F0 quantify and optimize microbial inactivation, while simultaneously protecting the load from unnecessary heat exposure.
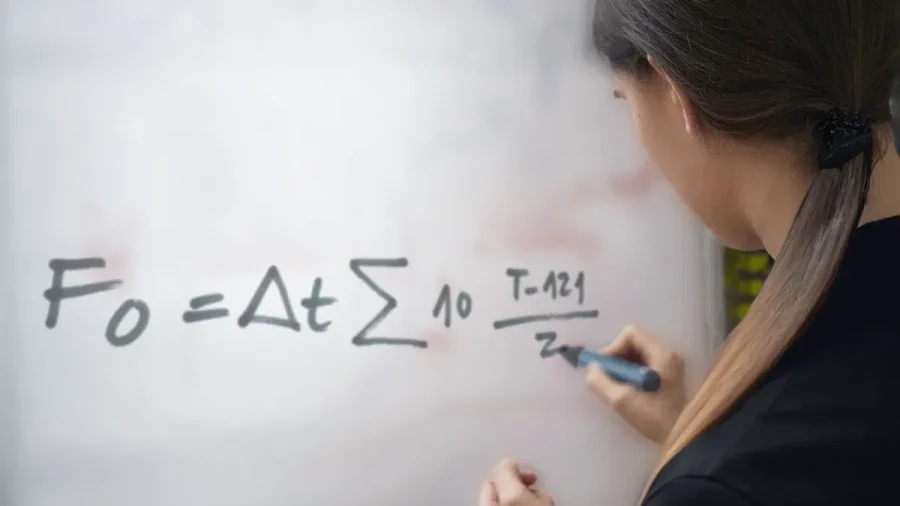
Sterilization plays a crucial role in various branches of the life sciences, being particularly vital in sectors such as pharmaceuticals, food industry, and microbiology. In this context, the F0 cycle in autoclaves marks a significant advancement as it allows the professionalization of sterilization processes, making it indispensable for experienced users working under high-quality standards.
This method not only ensures the complete inactivation of any form of life but also enables the quantification and evaluation of the efficiency of a sterilization process. It facilitates the comparison of the efficacy of different sterilization cycles at various temperatures and even between methods employing different sterilization technologies.
The F0 cycle is based on the concept of thermal equivalence, where the F0 value represents the equivalent minutes of sterilization at 121.1°C. For instance, a sterilization cycle with a F0 of 3 indicates a process equivalent to subjecting a load to 121.1°C for 3 minutes. Moreover, this same F0 of 3 can be achieved by sterilizing for 12 minutes at 115°C or 5 minutes at 119°C. Therefore, 3 minutes at 121°C is equivalent to 12 minutes at 115°C or 5 minutes at 119°C.
This approach makes it possible to quantify the sterility of a load and adjust the sterilization process according to the particular needs of what is being sterilized. Additionally, when an F0 autoclave is used together with a central flexible probe, the temperature inside the load can be measured and a sterilization process can be regulated by the F0 value obtained in the load itself and not by the chamber temperature, avoiding errors of lack of efficacy due to too short exposure times.
The great versatility of the F0 cycle is very advantageous in contexts where exposure to high temperatures may compromise the integrity of the load. It is also especially useful in contexts where accuracy and recording of the degree of sterility achieved in each process is vital. Also in the sterilization of large volumes of liquids, since this type of loads take a long time to heat up and cool down, so if we run a program for F0=3, the autoclave will calculate the sum of all the F achieved in each second, adding all the area un the curve, and will end the cycle when reaching the equivalent exposure of 3 minutes at 121.1°C but thanks to this thermal equivalence, it will achieve this F0=3 without ever reaching 121.1°C at any time.
If at some point you got lost, don’t worry, understanding the concept of F0 is not a simple matter. Below we will explain everything you need to know about the F0 value and F0 autoclaves.
Principles of F0 autoclaves
Sterilization using F0 in autoclaves is a process that integrates science, precision, and technology to effectively eliminate microorganisms. This method is grounded in fundamental physical principles related to energy transfer between two bodies and ensures the safety and efficacy of scientific experiments and production processes across a wide range of applications and industrial sectors. Below, we explain some of these basic principles:
Concept of F0
The heart of F0 sterilization is the F0 value, a parameter that quantifies the lethality of a sterilization process. It is defined as the equivalent exposure time at a reference temperature of 121.1°C required to achieve a specific level of sterility. This concept allows for the standardization and comparison of different sterilization cycles, ensuring they all achieve an equivalent level of sterility.
F0 formula
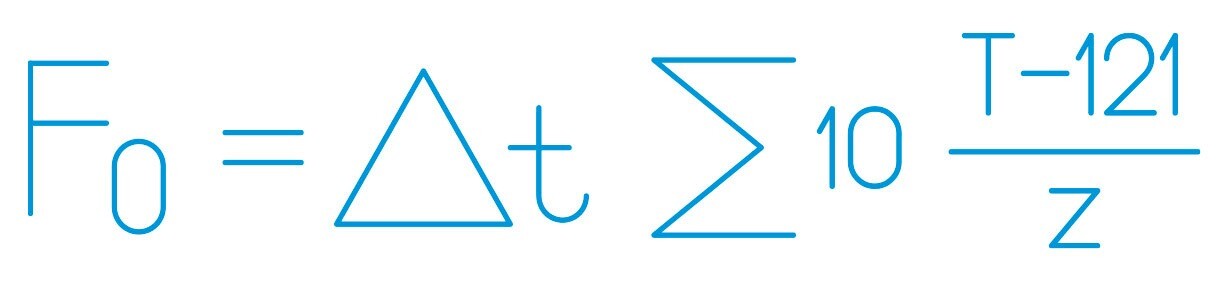
Δt = time interval between two successive measurements of T
T = temperature of the sterilized product at time t
z = temperature coefficient, typically assumed to be 10°C
Although the theoretical and mathematical justification of the F0 formula is beyond the scope of this post, two related concepts must be understood: the D-value and the Z-value.
- D-value, called decimal reduction time. Indicates the thermal susceptibility of a microorganism at a constant temperature. It is defined as the time required to destroy 90% of the microorganisms in a sample. For example, a D=1 corresponds to a 90% reduction, a D=3 to a 99.9% reduction, and a D=6 to a 99.9999% reduction. Typically, a D=1 is used, so it does not usually appear in the formula.
- Z-value, known as thermal resistance factor. This value shows how the inactivation of a specific microorganism varies with changes in the process temperature. As one might imagine, the inactivation caused by a sterilization process at 120°C for one minute differs significantly from that at 110°C for the same period.
Importance of temperature and time
In F0-controlled sterilizations, temperature and time are interdependent variables conditioned by the reference temperature and the maximum exposure temperature of the process. Thus, an F0 sterilization cycle offers a wide range of configuration possibilities.
In any case, higher temperatures require less time to reach the same F0 value, and vice versa. This relationship is crucial for adjusting sterilization cycles according to the specific needs of materials or products without compromising process efficacy.
Calculation in real time
F0 autoclaves are equipped with microprocessors that allow real-time calculation of the achieved F0 value every second. These systems continuously monitor the temperature within the autoclave and/or within the load, adjusting the sterilization cycle to ensure the desired F0 value is reached according to selected preferences. This capability is particularly useful for mitigating variations in the load or operational conditions of the autoclave.
Effective and safe sterilization
By using the F0 value to govern the sterilization cycle, autoclaves can ensure effective sterilization in any scenario. This is crucial in environments where ensuring the complete sterility of the load is essential, such as hospitals, laboratories, and the production of food and pharmaceuticals.

Advantages of the F0 cycle in autoclaves
F0 autoclaves represent a significant advancement in sterilization technology, offering multiple advantages over traditional methods. These benefits not only enhance the efficiency and effectiveness of the sterilization process but also contribute to greater safety and adaptability in various industrial and healthcare environments. Some of the main advantages of F0 autoclaves include:
-
Improved accuracy and precision in sterilizations
One of the major advantages of F0 autoclaves is their ability to quantify the lethality of a sterilization process with high precision and accuracy. By utilizing the F0 value, these autoclaves can adjust time and temperature to ensure the desired level of sterility is achieved, regardless of environmental or load variations.
-
Greater flexibility in handling different loads
F0 autoclaves are exceptionally adaptable to different types of loads. They can effectively sterilize a wide range of materials, from medical instruments to pharmaceuticals and food products, by automatically adjusting sterilization parameters for each type of load to ensure consistent sterility.
-
Efficacy and flexibility across a wide range of scenarios
Thanks to the precise control of temperature and time, F0 autoclaves are effective in a wide variety of scenarios, including those requiring gentler temperatures due to the thermolability of the load, such as food items, or higher temperatures for faster processes where the load can withstand greater heat exposure.
-
Time and energy savings
By optimizing sterilization cycles based on the F0 value, these autoclaves can significantly reduce the total sterilization time. This not only saves time but also reduces energy consumption, thereby lowering operational costs and minimizing environmental impact.
-
Enhanced process safety
The ability to monitor and adjust the sterilization process in real time significantly enhances safety. This is particularly important in environments where sterility is critical, such as healthcare settings and industry, as it reduces the risk of human error and inconsistencies in how each user loads the autoclave in terms of disposition and quantity of processed items.
-
Record keeping and documentation
Modern F0 autoclaves often include advanced recording and documentation capabilities, which are essential for complying with industry regulations, ensuring high-quality processes, and maintaining accurate records for process validation and audit purposes.

Automation of F0 sterilization in autoclaves
The automation of F0 autoclaves has revolutionized the sterilization process, elevating it to new levels of efficiency and reliability. This automation not only simplifies the sterilization process but also ensures greater consistency and precision in each batch processed.
F0 autoclaves are equipped with automated systems that precisely control and adjust the temperature and sterilization time. This ensures that each sterilization cycle achieves the desired F0 value, regardless of variations in load or operating conditions. This ability to calculate the F0 in real time allows the autoclave to make automatic adjustments during the cycle. If the system detects that the accumulated F0 is lower than the target, it will prolong the cycle to ensure effective sterilization. Conversely, if the system detects that the target F0 has already been achieved, it will terminate the sterilization phase.
Additionally, many F0 autoclaves can be integrated with centralized management systems, enabling remote control and monitoring, as well as the collection, recording, and analysis of data for continuous process improvement.
Practical applications of F0 autoclaves
Sterilization using F0 autoclaves represents not only a technical achievement in microbiology and sterilization but also has extensive and crucial practical applications across various sectors. These applications underscore how this technology has become an indispensable component in numerous environments. Below, we explore some of the most prominent practical applications:
-
Medical and hospital settings
In hospitals and clinics, F0 autoclaves are employed to sterilize surgical instruments, medical equipment, and other materials. The precision and efficacy of F0 sterilization ensure that these instruments are safe for use in medical procedures, thereby reducing the risk of infections and enabling professional traceability of each batch.
-
Research and biotechnology laboratories
Laboratories that work with cell cultures, biological samples, and pathogens rely on sterilization to maintain a contaminant-free environment. F0 autoclaves provide the necessary safety to conduct research and experiments in a sterile setting. A practical example in this sector is the preparation of large quantities of culture media, which may be demanded at varying scales. Thanks to F0 autoclaves, a consistent sterilization program can be executed regardless of the volume, whether sterilizing 5 bottles of 1L or 20 bottles of 1L. The program ensures that both processes achieve the same level of sterility, reducing the risk of contamination due to inadequate sterilization due to load variability ebtween different rotations.
-
Pharmaceutical industry
The production of pharmaceuticals requires stringent control over the sterility of each batch to ensure the safety and efficacy of the products. F0 autoclaves are used to sterilize raw materials used in drug manufacturing, as well as finished forms and packaging. This technology also facilitates continuous improvement and automatic recording of all processes, enhancing quality control.
-
Food industry
In food production, particularly in preservation and packaging, sterilization is essential to prolong the shelf life of products and prevent spoilage. F0 autoclaves are critical in ensuring that packaged foods are free from microorganisms while preserving the organoleptic properties of the finished products. These autoclaves are often equipped with fast cooling systems. By employing sterilization cycles regulated by F0, unnecessary overcooking of foods is avoided. Once the target F0 value is reached, fast cooling begins automatically, efficiently cooling the load and ensuring the quality of the sterilized food.