Autoclaves > Top line verticals TLV-S-MD Series
Multipurpose vertical medical autoclaves
The TLV-S-MD Series vertical medical autoclaves are designed for the sterilization of both solid and liquid loads.
- Fully automatic, equipped with cutting-edge engineering and exceptional quality in every detail
- Modern design with 7'' capacitive touchscreen display
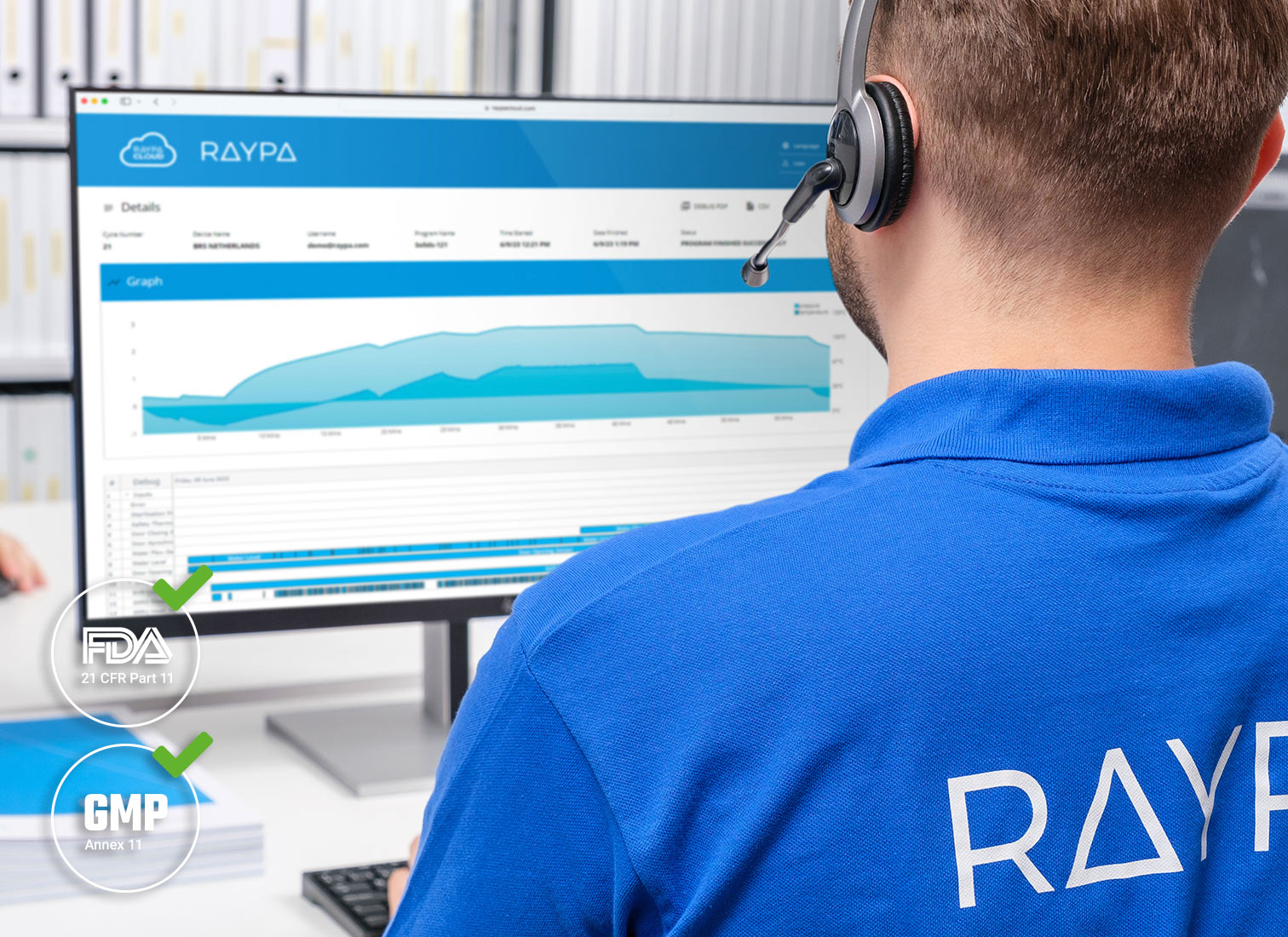
- Advanced cloud-based connectivity that complies with FDA, GMP, and GLP standards
Multipurpose vertical medical autoclaves
Multipurpose autoclaves for solids and liquids designed to meet the sterilization needs of healthcare environments in the medical industry.
Top line vertical autoclaves enable fully digital operation. All models are equipped with the most advanced connectivity on the market and comply with the latest advances in electronic records and data control to be able to work under FDA, GMP and GLP environments.

State-of-the-art sterilization technology
State-of-the-art technology. Push-button-controlled mechanically assisted door. Instant steam production by the built-in steam generator. Air inlet and outlet fitted with a bacteriological filter. Built-in vacuum pump for initial prevacuum. F₀-controlled sterilizations thanks to the integrated flexible probes. Automatic filling from the water network. Water-cooled direct discharge.
Modern design with a 7'' touchscreen display
Large capacitive screen, control over all parameters, real-time program visualization, admin user control, and access to protocol history. Includes multiple video tutorials and a ticket builder.
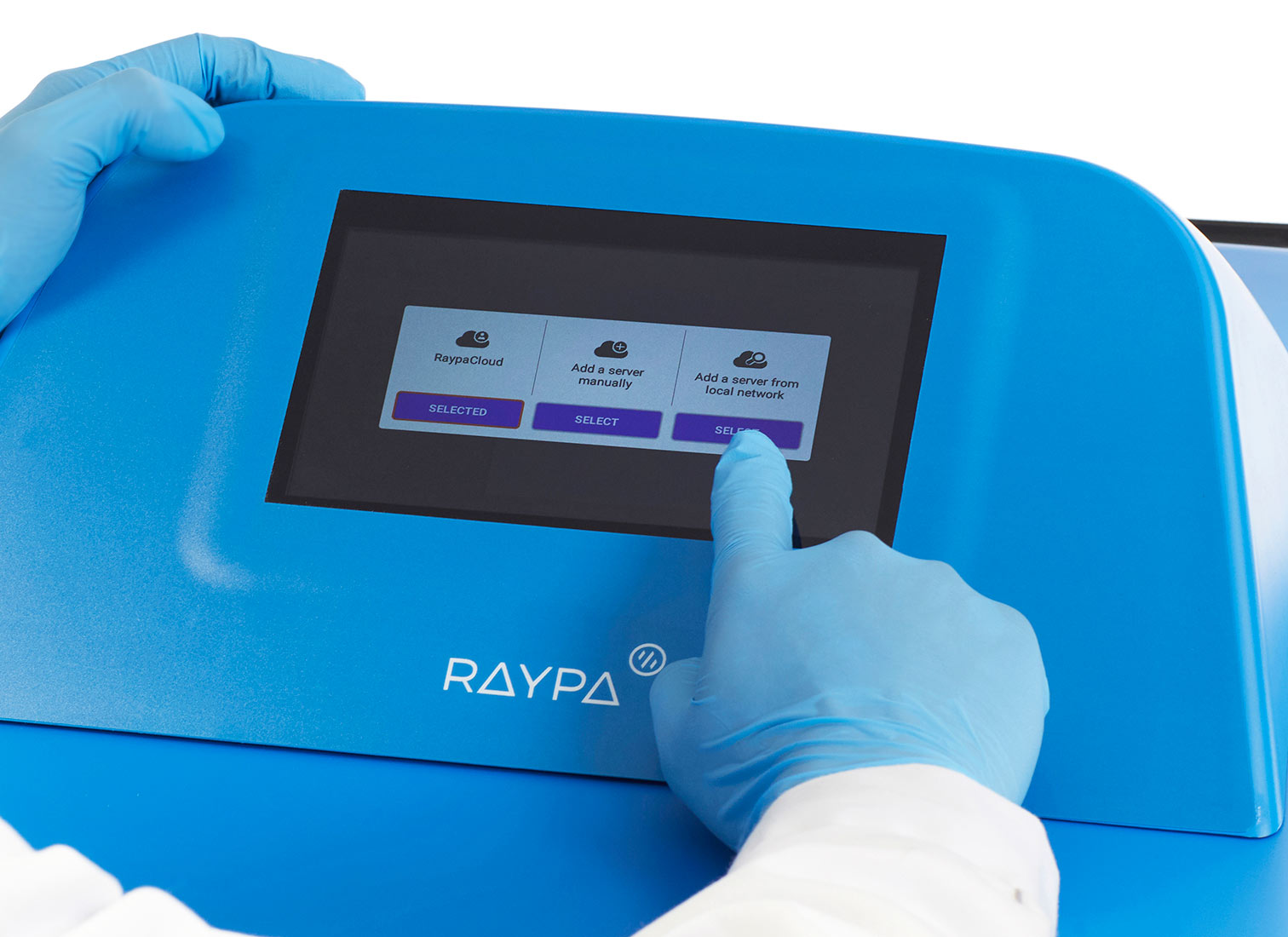
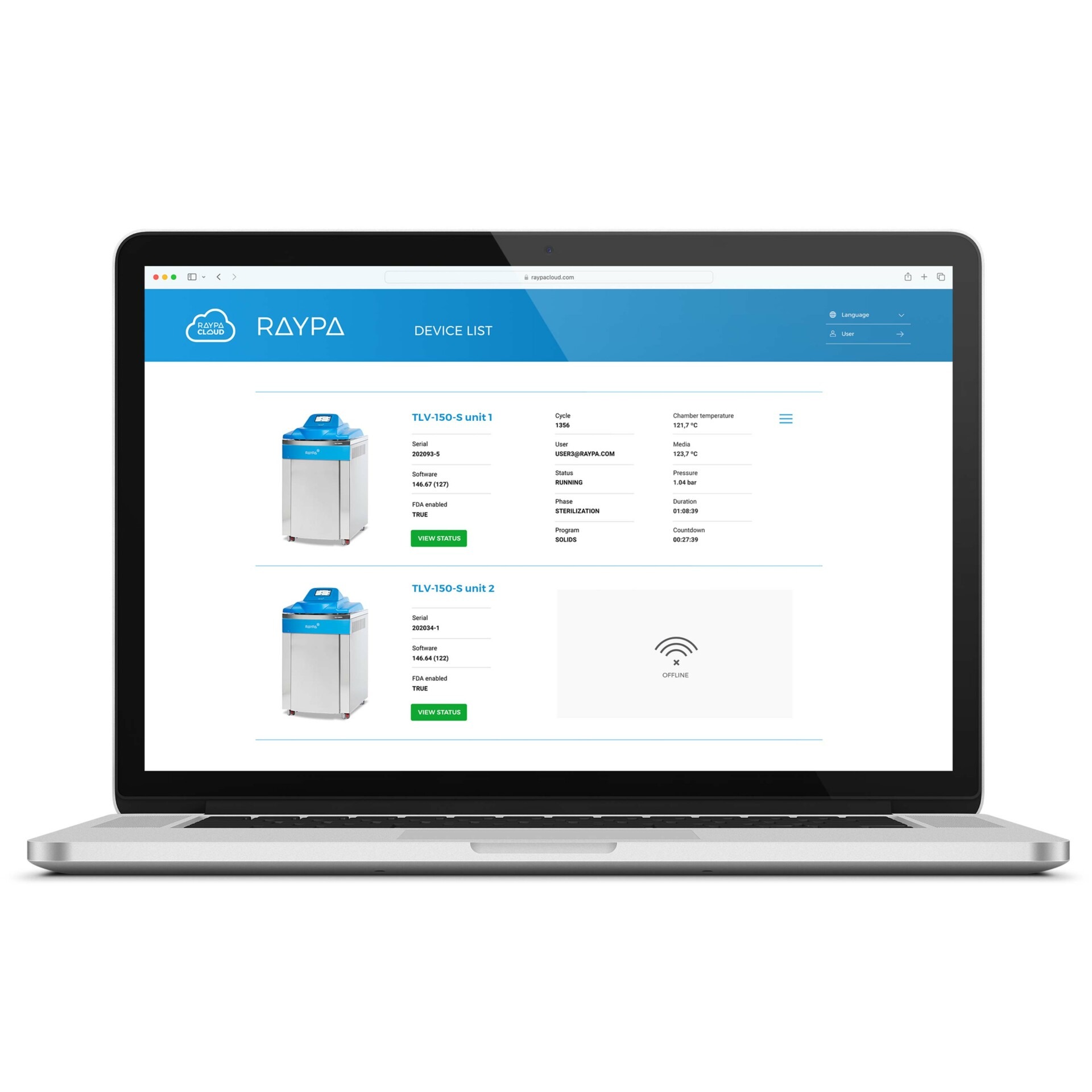
Cloud-based connectivity and advanced technical support
Advanced technical support: Book and request technical assistance directly from the controller, use TeamViewer® for screen sharing, and authorize a remote diagnosis of the equipment's status. Additionally, enjoy centralized management for several autoclaves with tailored reports, handle notifications across multiple devices, and ensure automatic traceability for audits, all protected with SSL encryption. The cloud-based management software is adaptable for integration on a private server through Docker, Active Directory, or LIMS.
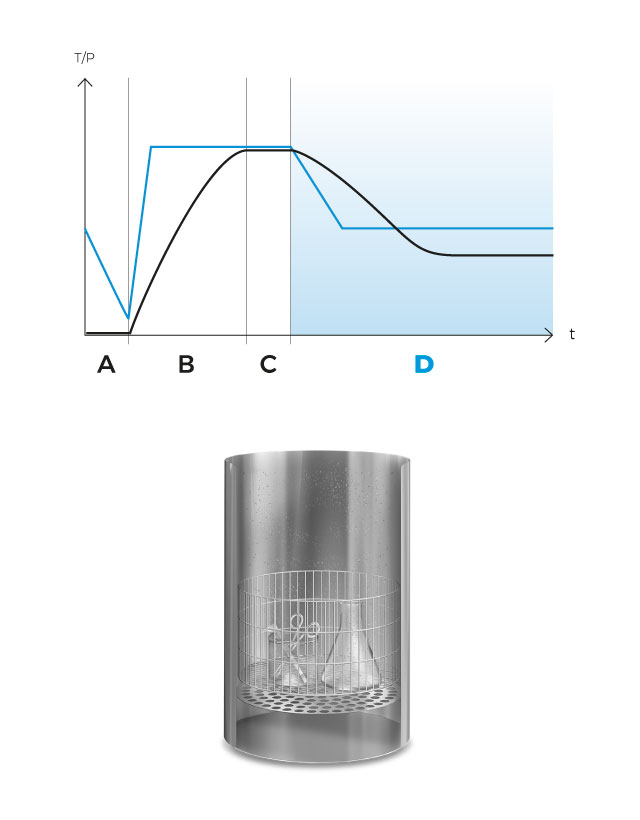
Operation
In the pre-vacuum phase the air in the chamber is mechanically purged by means of a vacuum pulse. The steam generator is then activated, which will inject steam into the sterilization chamber. When the sterilization temperature is reached, the sterilization phase begins and the temperature is accurately maintained during the sterilization time.
At the end of the sterilization phase, a natural cooling phase begins. In programs with Agar mode, the preset temperature will be maintained indefinitely.
Temperature
Pressure
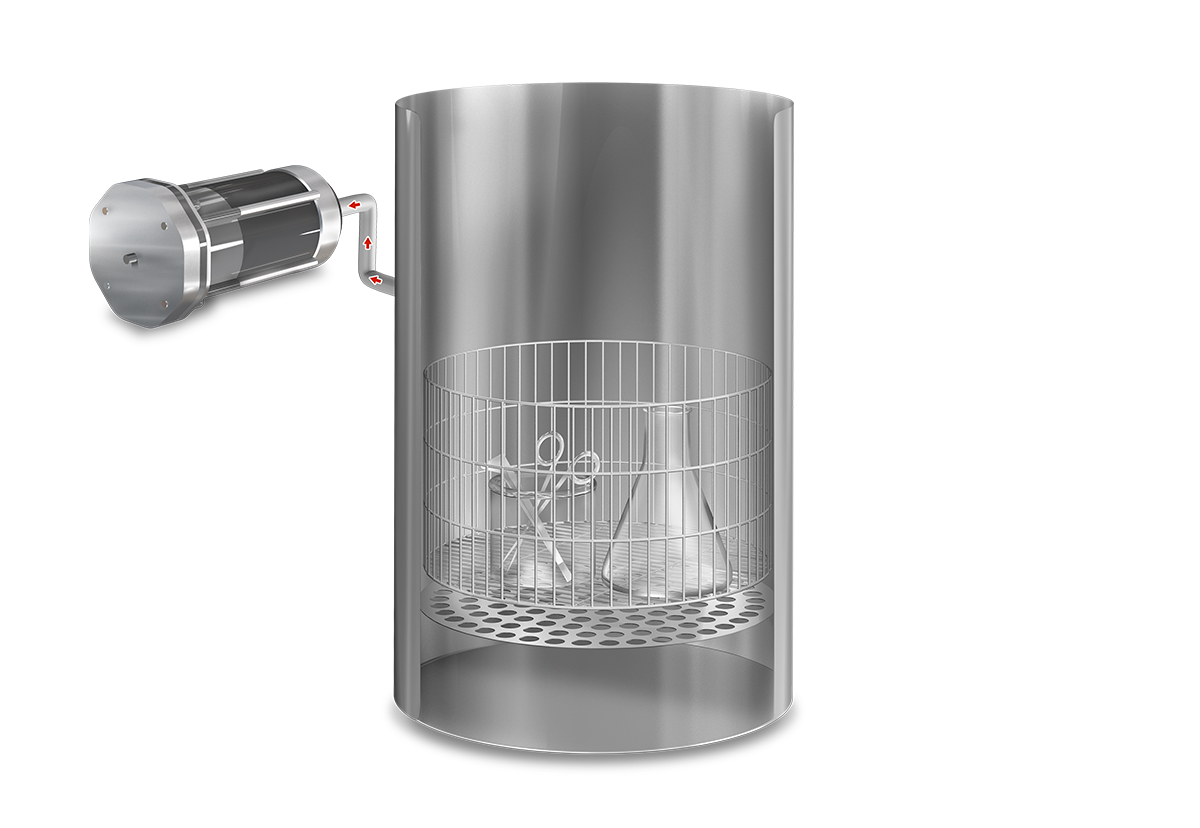
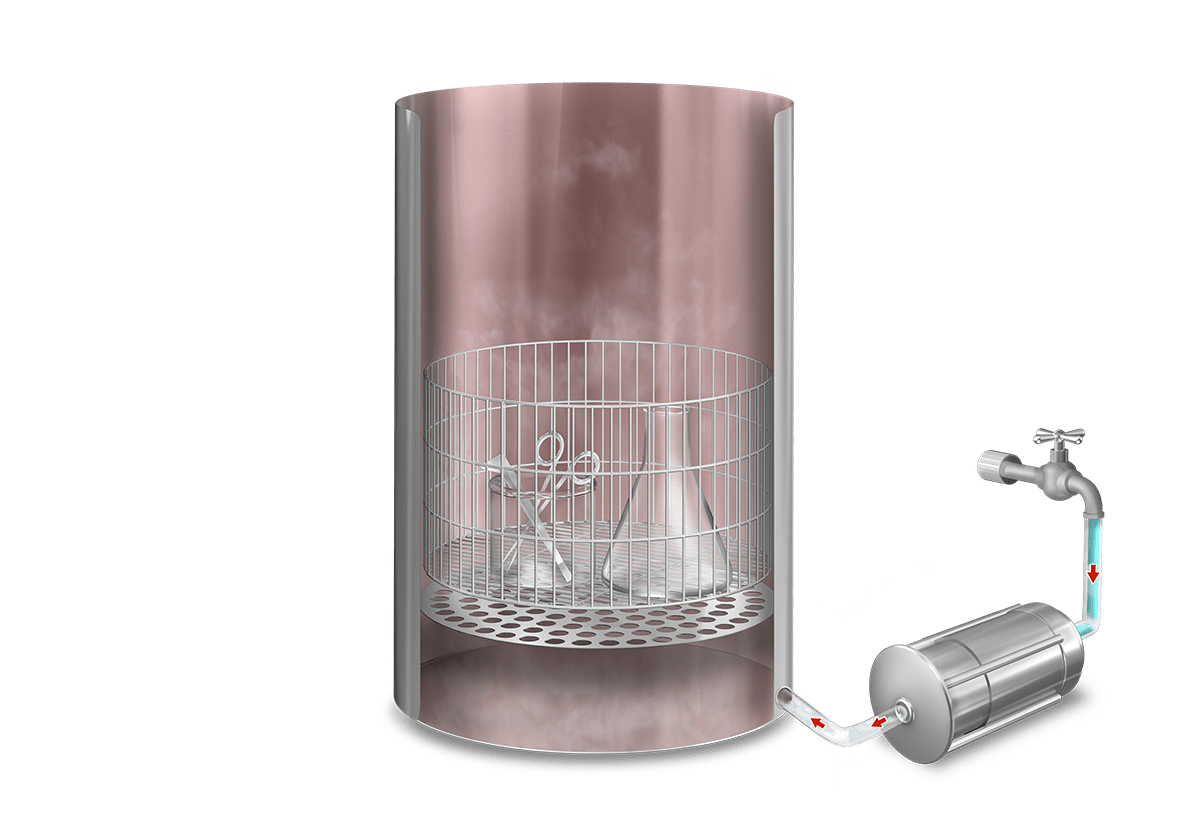


Operation
In this phase the cold air in the chamber is mechanically purged to the outside by means of a vacuum pulse, reducing the presence of non-condensable gases and facilitating the penetration of steam throughout the load.
Temperature
Pressure
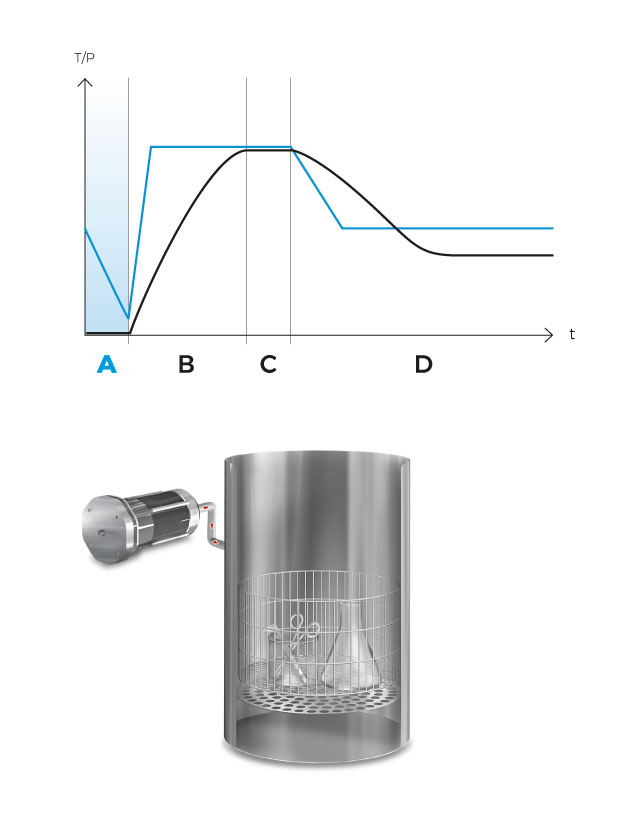
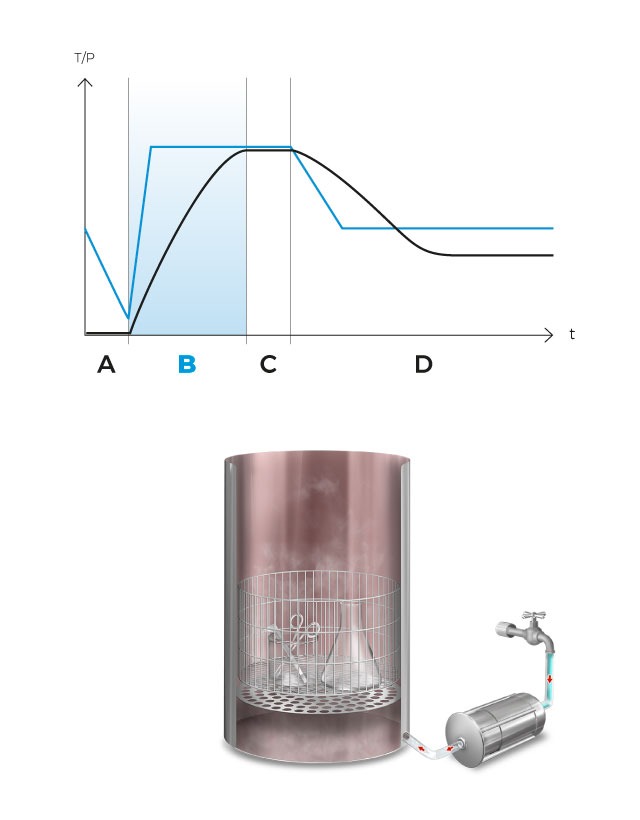
After completing the first vacuum pulse, the steam generator is activated and will inject saturated steam into the sterilization chamber until the sterilization temperature is reached.
Temperature
Pressure
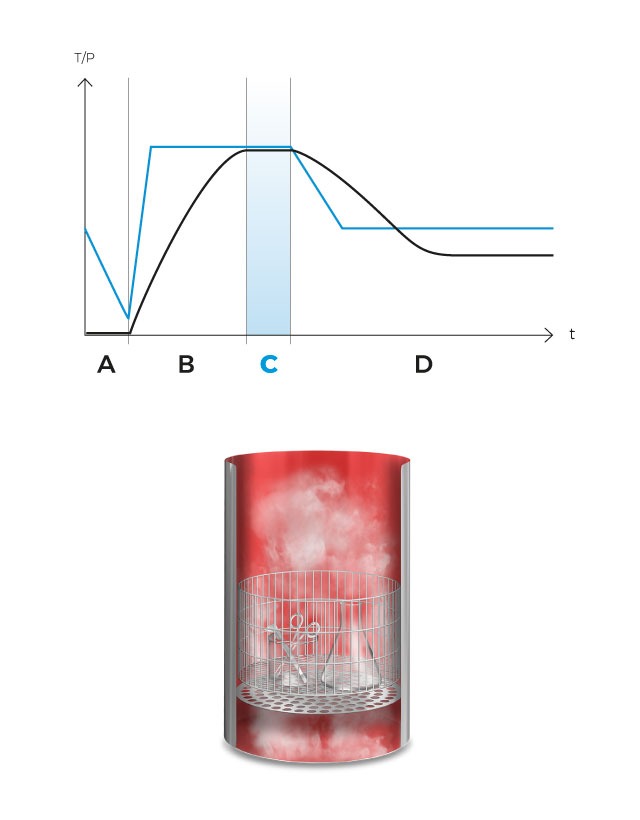
Upon reaching the sterilization temperature in the medium, the sterilization phase begins and the temperature is accurately sustained for the predefined duration. Antibiotics, pH correctors or thermolabile ingredients can be dispensed at any time using the external dispensing ports.
Temperature
Pressure
Finally, a natural cooling phase begins until a safe temperature is reached. Optionally, the agar mode can be configured to maintain a constant temperature.
Temperature
Pressure
Applications

LIQUIDS AND CULTURE MEDIA
Sterilize your liquid loads professionally using F₀ and a flexible probe. In addition, you will be able to do it comfortably and without rushing to remove the load at the end thanks to the agar mode.

PLASTICS AND METAL OBJECTS
Sterilize plastics and metal objects conveniently with faster cycles thanks to the steam generator.

GLASSWARE
Ensure the purity of your glass instruments thanks to the steam generator that produces high-quality steam, which minimizes salt transfer during processing.

SANITARY WASTE
Thanks to the initial prevacuum pulse and the steam generator, you can sterilize waste bags without any problems.

BIOHAZARDOUS WASTE
The bacteriological filter at the air inlet and outlet of the chamber allows the processing of biohazardous loads.
Technical data
| References |
|---|
| Total/usable volume of the chamber (L) |
| Usable dimensions of the chamber (Ø x H mm) |
| External dimensions (L x D x H mm) |
| Loading height (mm) |
| Net weight (Kg) |
| Power (W) |
| Voltage* (V) |
| Frequency (Hz) |
| TLV-50-MD |
| 58/56 |
| 400 x 450 |
| 610 x 870 x 1060 |
| 815 |
| 131 |
| 3600 |
| 230 |
| 50/60 |
| TLV-75-MD |
| 83/81 |
| 400 x 650 |
| 610 x 870 x 1110 |
| 865 |
| 139 |
| 3600 |
| 230 |
| 50/60 |
| TLV-110-MD |
| 124/118 |
| 500 x 600 |
| 710 x 980 x 1160 |
| 915 |
| 195 |
| 9000 |
| 400 |
| 50/60 |
| TLV-150-MD |
| 169/155 |
| 500 x 850 |
| 710 x 980 x 1310 |
| 1065 |
| 210 |
| 9000 |
| 400 |
| 50/60 |
| *Other voltages and electrical configurations available on request. |
Accessories
RAYPACLOUD
License to access the cloud-based management platform, enabling all remote connectivity and diagnostic functions.

Activation of FDA on the controller
Activates all the necessary functions to comply with FDA and GMP regulations: audit trail, automatic backups, electronic signatures, etc.

Flexible probe
Adds a flexible probe for improved sterilization of liquid cargoes

Integrated crane
Mobile crane integrated into the side of the autoclave, designed to assist in the loading and unloading of heavy items.

Independent crane
Crane with integrated battery, mobile and independent, designed to assist in the loading and unloading of heavy items.

Wire baskets
Baskets suitable for the sterilization of all types of clean loads.

Wire basket tray
Tray designed to be used in conjunction with wire baskets to collect liquids.

Unperforated baskets
Baskets suitable for sterilization of dirty loads and those at risk of spillage.

Schimmelbusch Drum
Drum suitable for sterilization of medical instruments and biohazardous loads.

Height adjustable tray support
For sterilization of instruments, small bags and other small objects that must be placed straight up.

Integrated ticket printer
Prints program number, cycle number, temperature, date and hour of the run and error messages.

Desktop receipt printer
Prints program number, cycle number, temperature, date and hour of the run and error messages.

Label printer
Allows printing of individual labels with customizable barcode design according to GLP.

Bar code scanner
Allows to read individual labels of each processed load and identify each batch according to GLP.

Eco-efficient water purifier
Eco-efficient direct-flow water purifier without water storage, capable of filtering 1.3L/min with an LED display.

Purified water tank
25L bottle for storing purified water, which will be used to supply the autoclave. This is to be used in conjunction with our water purifier.

Transport trolley
Auxiliary trolley to assist in loading and unloading the autoclave.

Cable gland
Cable gland allowing access for up to 8 probes for calibration and validation operations.

Sterilization control tape
Class 1 indicator for steam sterilization.

IQ-OQ Documentation
Delivery of documentation and IQ/OQ protocol for third party autoclave qualification.

IQ-OQ-PQ qualification
IQ/OQ/PQ autoclave qualification service performed by RAYPA.

FDA validation of the controller
Validation service to ensure the controller complies with FDA regulation 21 CFR Part 11.

FDA validation of the cloud connectivity
Validation service ensuring that cloud-based or private server connectivity complies with FDA regulation 21 CFR Part 11.

Private server
Private cloud server communicating with the autoclave through the local laboratory network with optional FDA and GMP compliance.

On-site commissioning & training
On-site guided start-up that includes the verification of the correct operation and installation of the equipment, complemented by a training session on its operation and maintenance for users.

Remote commissioning & training
Remote start-up including a training session on the use and maintenance of the equipment.

Maintenance contract
A schedule of regular inspections that encompasses technical assessments, probe calibration, and adherence to the preventative maintenance plan, along with discount rates.

Set of consumables, spare parts and essential components
Set of original spare parts, consumables, and components, chosen specifically to adhere to each model's maintenance plan, intended to maximize equipment longevity and minimize downtime in the event of a malfunction.

Warranty extension
Extended warranty up to a total of 3 years
You may also be interested in
Vertical medical autoclaves with super-drying system
- TLV-PD-MD Series
- Top Line
Vertical medical autoclaves with fast cooling system
- TLV-FA-MD Series
- Top Line
Subscribe to our newsletter
"*" indicates required fields