Autoclaves > Top line TLV-MP Series
Expert media preparator
The TLV-MP Series media preparators are designed for the production of large volumes of culture media in industrial settings operating under GLP, GMP and FDA environments.
- Up to 95% faster production of culture media
- Functions as both an autoclave and a media preparator, providing cost and space savings
- Advanced cloud-based connectivity that complies with FDA, GMP, and GLP standards
Expert media preparator
The media preparators from the TLV-MP Series optimize the workflow of microbiology and plant tissue culture laboratories by saving on costs, preparation time, and workload.
A single device encompasses the preparation, sterilization, fast cooling, and dispensation of high-quality culture media with excellent batch-to-batch reproducibility. Its design prevents contamination, gelation, solubility, or homogeneity issues, as well as burns. Moreover, it provides complete traceability of all executed operations.
The TLV-MP Series media preparators are engineered to reduce the total preparation time and supply large volumes of culture media. Choose the setup that best suits your laboratory from among the 4 sizes of media preparators and the 4 available dispensing modes. Additionally, the heating capacity of each model can be upgraded to drastically reduce the duration of the heating phase.
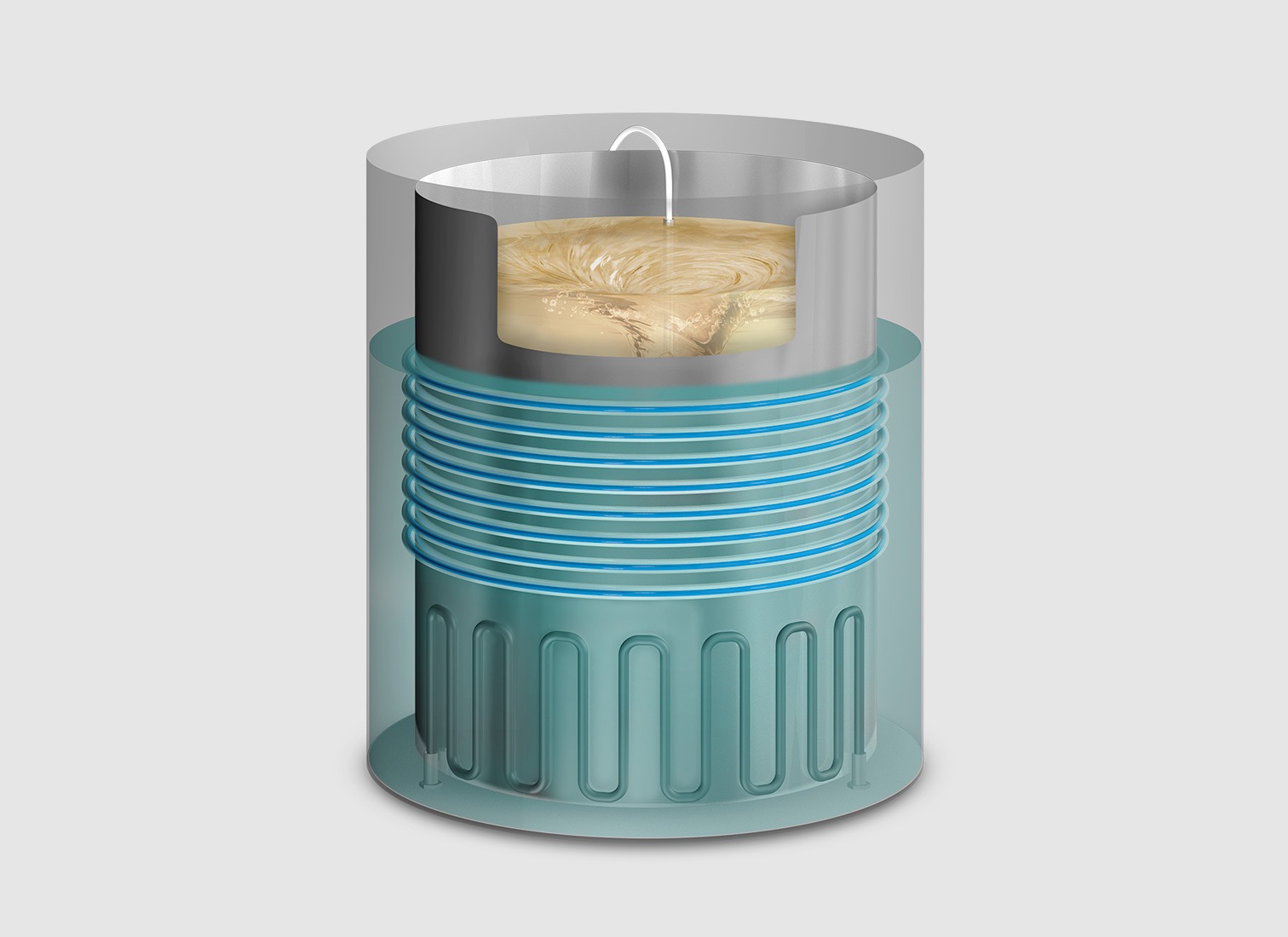
Streamline your preparation time, speed up dispensation and reduce workload
Enjoy much faster production times compared to the traditional autoclave method. Preparation volumes range from 36L to 90L, with dispensing speeds ranging from 7 mL/s to 100 mL/s. Select the dispensation temperature that best meets your requirements.
Modern design and hassle-free cleaning with multiple automated functions
Push-button-controlled mechanically assisted door. Automatic filling from the water network. Water-cooled direct discharge. Dispensing lines can be cleaned and disinfected before, during, and after the dispensing phase with steam that reach the entire length of the line to prevent contamination. Pause the dispensing phase to avoid gelation issues. The inner bucket features ergonomic handles and can be easily removed.


Advanced technical support
Book and request technical assistance directly from the controller, use TeamViewer® for screen sharing, and authorize a remote diagnosis of the equipment's status. Additionally, enjoy centralized management for several media preparators with tailored reports, handle notifications across multiple devices, and ensure automatic traceability for audits, all protected with SSL encryption. The cloud-based management software is adaptable for integration on a private server through Docker, Active Directory, or LIMS.
Operation
Temperature
Pressure
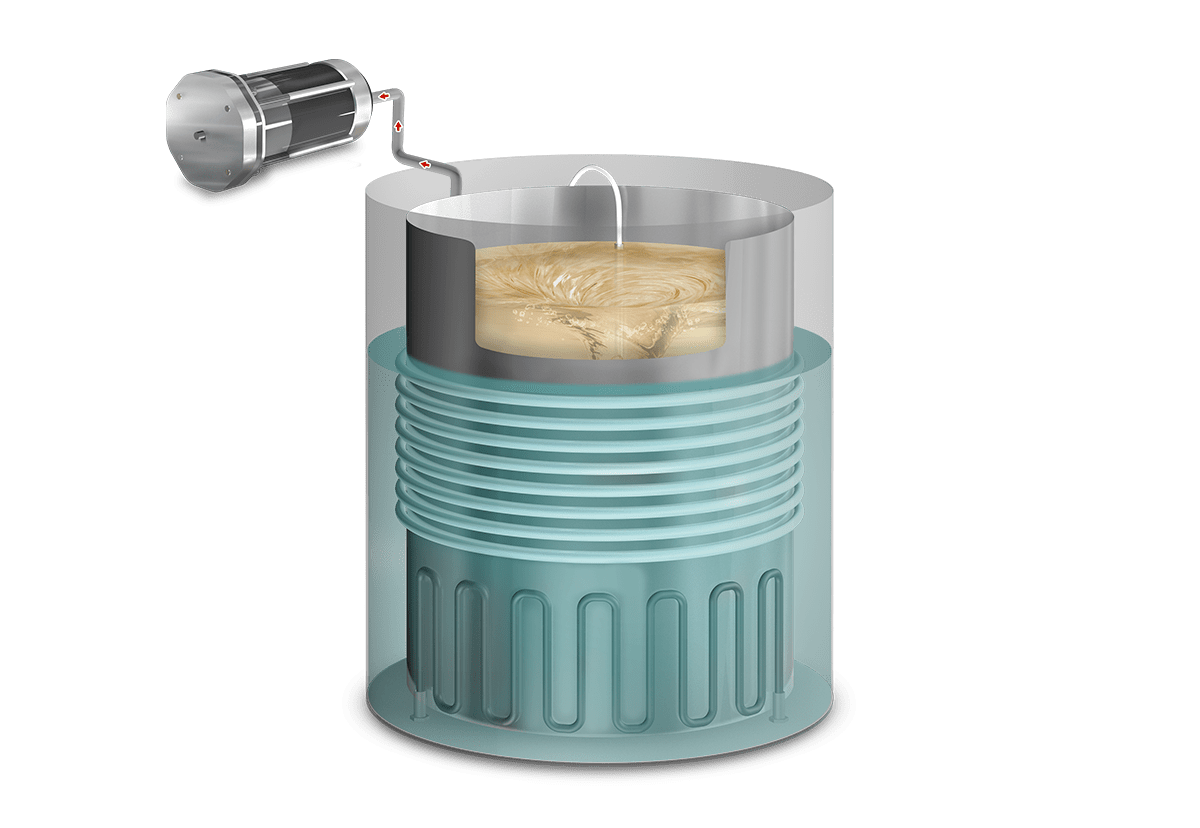


Operation
In this phase, the cold air from the chamber is mechanically purged to the outside through a vacuum pulse, reducing the presence of non-condensable gases and facilitating the heating of the media.
Temperature
Pressure
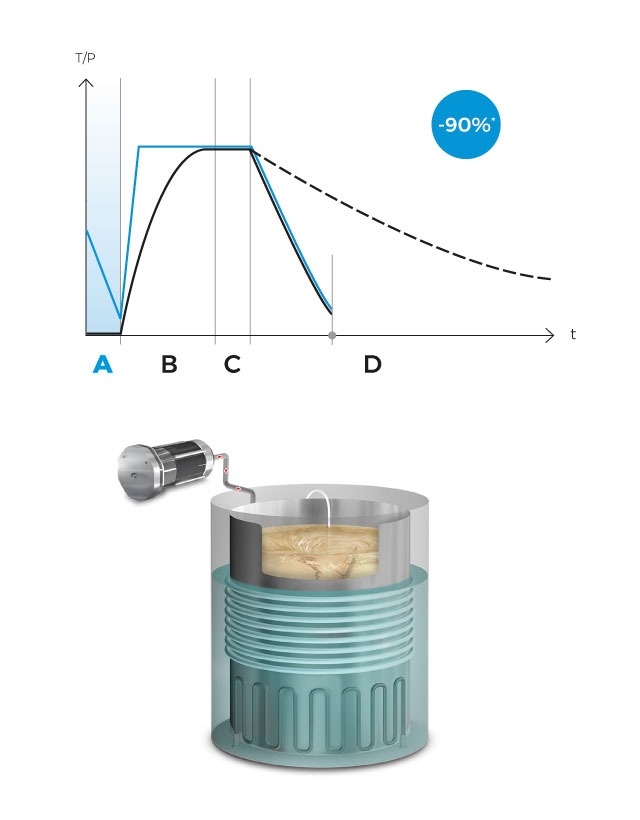
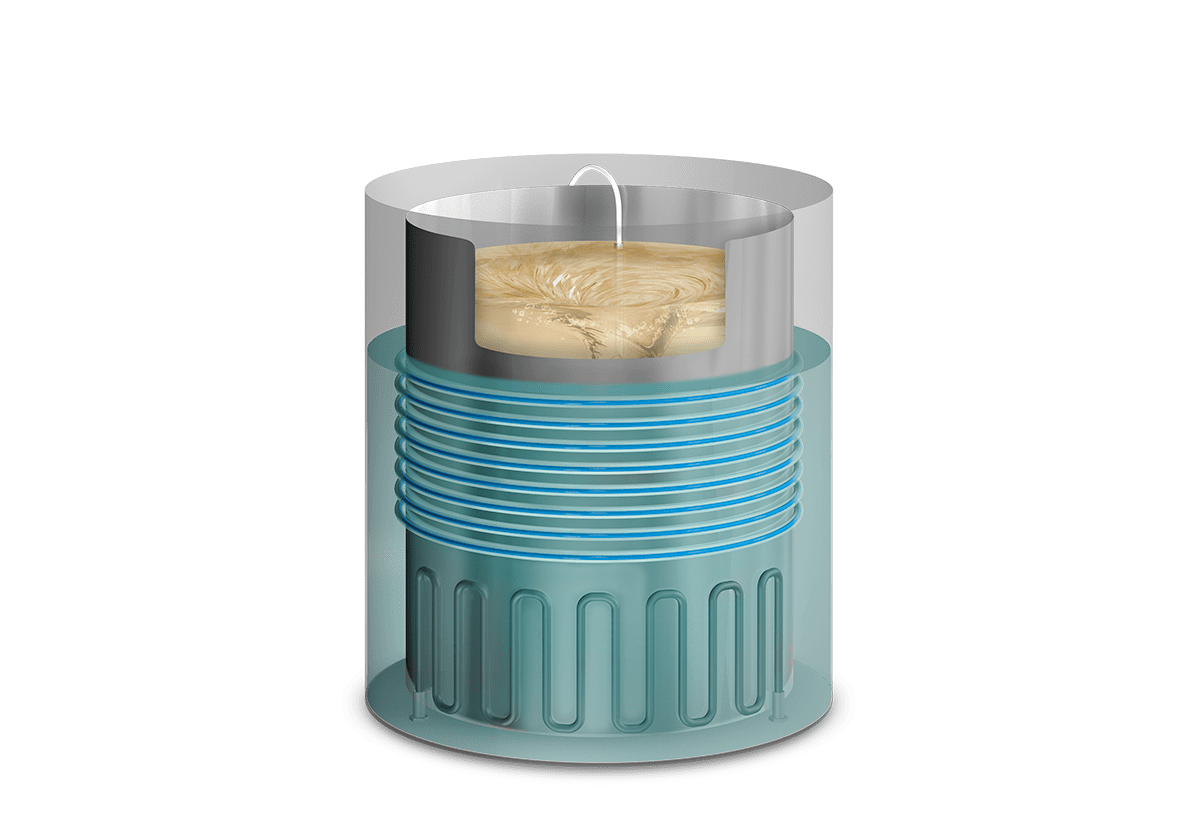
The powerful heating elements of the sterilization chamber heat up water to produce saturated steam and warm the culture media located inside the inner vessel. Simultaneously, the magnetic stirrer of adjustable stirring speed, ensures the correct dissolution of the solute in the solvent.
Temperature
Pressure
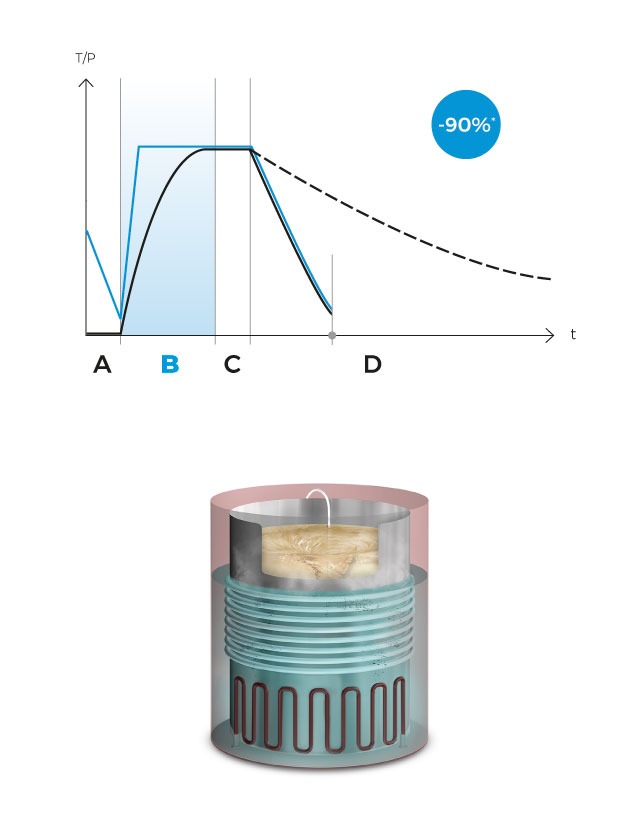
Upon reaching the sterilization temperature in the medium, the sterilization phase begins and the temperature is accurately sustained for the predefined duration.
Temperature
Pressure
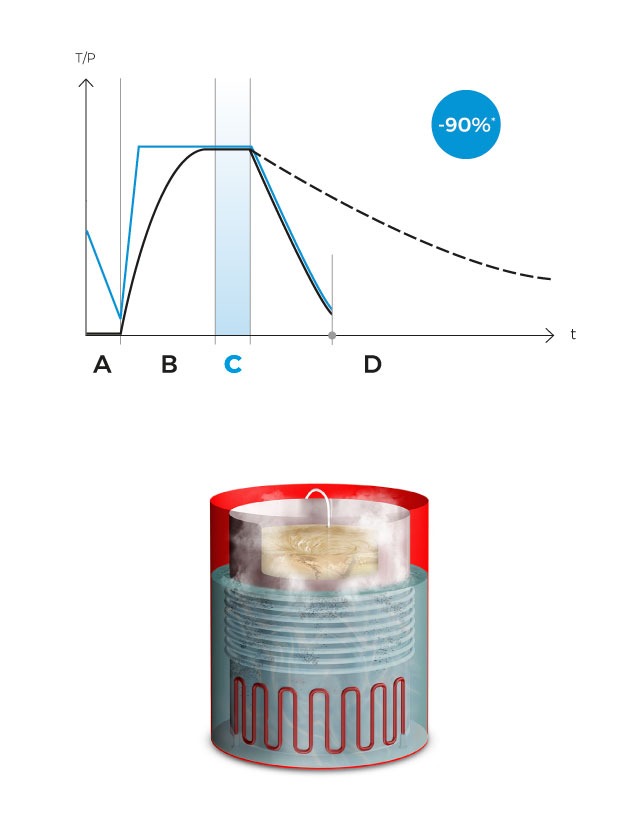
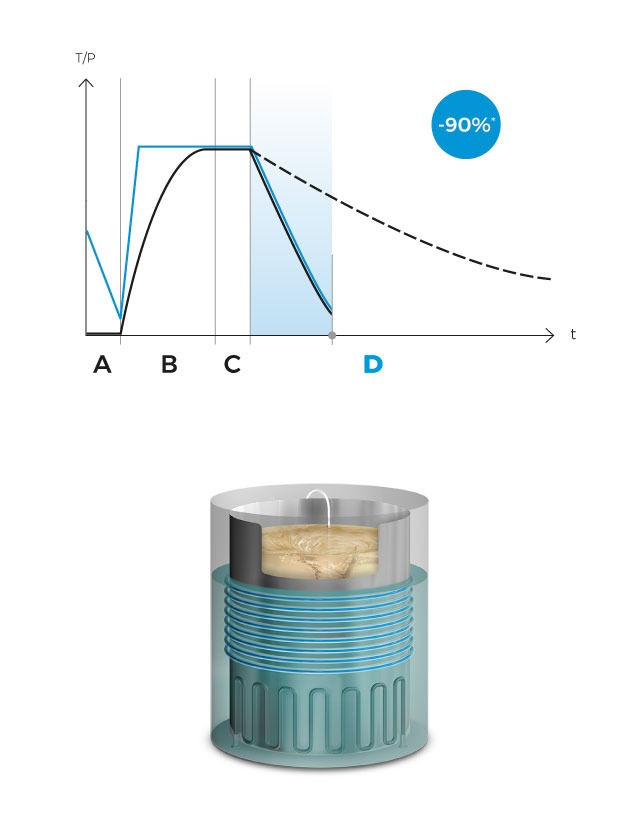
Finally, a fast cooling phase begins with pressure support, activating the water coils that surround the sterilization chamber to quickly cool the load until it reaches the dispensation temperature, which will be maintained indefinitely until all the prepared culture medium is dispensed. Antibiotics, pH correctors or thermolabile ingredients can be dispensed at any time using the external dispensing ports.
Temperatura
Presión
Applications
Preparation of agar
The fast cooling system saves a lot of time. Additionally, you can start dispensing the sterilized medium whenever you want since the temperature is maintained at the chosen temperature indefinitely. Likewise, the dispensing process can be interrupted and resumed later thanks to the pressure support system, preventing the agar in the dispensing lines from solidifying.

Plant tissue culture
Thanks to dispensation through pressure support, you can dispense large volumes at high speed. Additionally, larger models can be overpowered, which, along with fast cooling, allows for a drastic reduction in the duration of each cycle. pH correctors can be dispensed at any time using the external dispensing ports.

Preparation of buffer solution
Thanks to dispensation through pressure support, you can dispense large volumes at high speed. Additionally, larger models can be overpowered, which, along with fast cooling, allows for a drastic reduction in the duration of each cycle. pH correctors can be dispensed at any time using the external dispensing ports.

Preparation of lysogeny broth
Thanks to dispensation through pressure support, you can dispense large volumes at high speed. Additionally, larger models can be overpowered, which, along with fast cooling, allows for a drastic reduction in the duration of each cycle.
Technical data
| References |
|---|
| Maximum capacity for preparing culture media (L) |
| Minimum capacity for preparing culture media (L) |
| External dimensions (L x D x H mm) |
| Inner vessel dimensions (Ø x H mm) |
| Net weight (Kg) |
| Power (kW) |
| Voltage* (V) |
| Frequency (Hz) |
| TLV-40-MP |
| 36 |
| 5 |
| 750 x 980 x 1080 |
| 330 x 461 |
| 195 |
| 12 |
| 400 |
| 50/60 |
| TLV-60-MP |
| 54 |
| 10 |
| 750 x 980 x 1300 |
| 330 x 696 |
| 205 |
| 15 |
| 400 |
| 50/60 |
| TLV-80-MP |
| 72 |
| 20 |
| 850 x 1080 x 1200 |
| 420 x 594 |
| 238 |
| 20 or 30 |
| 400 |
| 50/60 |
| TLV-100-MP |
| 90 |
| 20 |
| 850 x 1080 x 1340 |
| 420 x 734 |
| 265 |
| 20 or 30 |
| 400 |
| 50/60 |
| *Other voltages and electrical configurations available on request. |
Accessories
RAYPACLOUD
License to access the cloud-based management platform, enabling all remote connectivity and diagnostic functions.

Activation of FDA on the controller
Activates all the necessary functions to comply with FDA and GMP regulations: audit trail, automatic backups, electronic signatures, etc.

Automatic system for dispensing culture media
Fast and automated system for dispensing culture media into Petri dishes.

Kit for use as autoclave
Set of racks and baskets made of AISI-304 stainless steel that allow the use of the media preparator as an autoclave.

Additional peristaltic pump
Second peristaltic pump to double the dispensing flow rate.

Dispensing lines
Silicone dosing tubes to increase dispensing speed.

External dosing station
Automates and speeds up the dispensing phase of repetitive operations.

Splitting of dispensing lines
Custom modification to divide the dispensing lines, enabling the supply of two distinct workstations from a single media preparator.

Eco-efficient water purifier
Eco-efficient direct-flow water purifier without water storage, capable of filtering 1.3L/min with an LED display.

Validation and qualification sets
Set of reader and temperature probes of specific length and diameter to perform the validation and qualification of media preparators.

External probe adapter
Adapter for external validation probe.
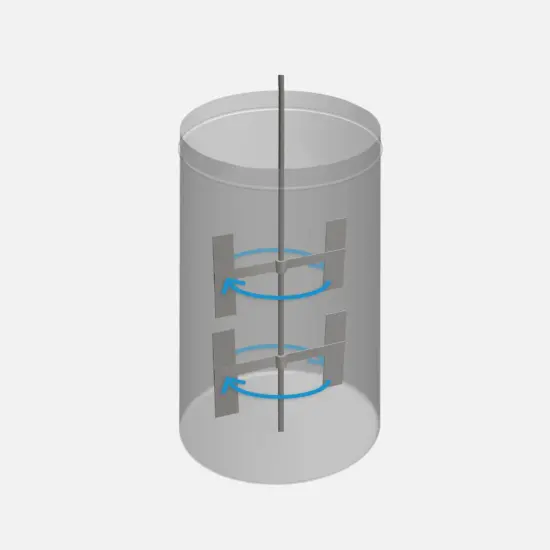
High viscosity paddle system
Installation of a tangential flow paddle system, designed for applications involving the processing of high-viscosity solutions.

Integrated thermal printer
It prints the program number, cycle and the evolution of parameters in the course of the cycle.

Desktop dot matrix printer
Prints program number, cycle number, temperature, time, date and time of each sterilization, and error messages.

Label printer
Allows printing of individual labels with customizable barcode design according to GLP.

Bar code scanner
Allows to read individual labels of each processed load and identify each batch according to GLP.

Automatic water filling
Water pump for automating the supply of the sterilization chamber with purified water.

Table for media preparators
Stainless steel table for placing benchtop media preparators or the CAR-MP dispensing system at an optimal height.

Transport trolley
Auxiliary trolley to assist in loading and unloading the autoclave.

IQ-OQ Documentation
Delivery of IQ/OQ documentation and protocol to perform a third-party qualification of the media preparator.

IQ-OQ-PQ qualification
IQ/OQ/PQ autoclave qualification service performed by RAYPA.

FDA validation of the controller
Validation service to ensure the controller complies with FDA regulation 21 CFR Part 11.

FDA validation of the cloud connectivity
Validation service ensuring that cloud-based or private server connectivity complies with FDA regulation 21 CFR Part 11.

Integration of the management platform on the client's local server
Integration of the management platform into a local area network via Docker.

Private server
Private cloud server communicating with the autoclave through the local laboratory network with optional FDA and GMP compliance.

Integration within Active Directory
Integration of administrator management, user management and password policies within Active Directory.

On-site commissioning & training
On-site commissioning, which includes verification of the correct operation and installation of the equipment and a training session for users on the use and maintenance of the equipment.

Remote commissioning & training
Guided remote start-up including a training session for users on the operation and maintenance of the equipment.

Maintenance contract
Regular inspection plan that includes technical inspection, probe calibration and compliance with the preventive maintenance plan, as well as tariff discounts, all performed by our expert technicians.

Set of spare parts and essential components
Set of spare parts, critical components and original consumables selected to comply with the maintenance plan of each model with the objective of maximizing the useful life of the equipment and minimizing downtime in case of failure.

Warranty extension
Extended warranty up to a total of 3 years
You may also be interested in
Standard media preparators
- AE-MP Series
- Classic Line
Subscribe to our newsletter
"*" indicates required fields























